ESHRE guideline: recurrent pregnancy loss
- PMID: 31486805
- PMCID: PMC6276652
- DOI: 10.1093/hropen/hoy004
ESHRE guideline: recurrent pregnancy loss
Abstract
Study question: What is the recommended management of women with recurrent pregnancy loss (RPL) based on the best available evidence in the literature?
Summary answer: The guideline development group formulated 77 recommendations answering 18 key questions on investigations and treatments for RPL, and on how care should be organized.
What is known already: A previous guideline for the investigation and medical treatment of recurrent miscarriage was published in 2006 and is in need of an update.
Study design size duration: The guideline was developed according to the structured methodology for development of ESHRE guidelines. After formulation of key questions by a group of experts, literature searches and assessments were performed. Papers published up to 31 March 2017 and written in English were included. Cumulative live birth rate, live birth rate and pregnancy loss rate (or miscarriage rate) were considered the critical outcomes.
Participants/materials setting methods: Based on the collected evidence, recommendations were formulated and discussed until consensus was reached within the guideline group. A stakeholder review was organized after finalization of the draft. The final version was approved by the guideline group and the ESHRE Executive Committee.
Main results and the role of chance: The guideline provides 38 recommendations on risk factors, prevention and investigations in couples with RPL, and 39 recommendations on treatments. These include 60 evidence-based recommendations - of which 31 were formulated as strong recommendations and 29 as conditional - and 17 good practice points. The evidence supporting investigations and treatment of couples with RPL is limited and of moderate quality. Of the evidence-based recommendations, only 10 (16.3%) were supported by moderate quality evidence. The remaining recommendations were supported by low (35 recommendations: 57.4%), or very low quality evidence (16 recommendations: 26.2%). There were no recommendations based on high quality evidence. Owing to the lack of evidence-based investigations and treatments in RPL care, the guideline also clearly mentions investigations and treatments that should not be used for couples with RPL.
Limitations reasons for caution: Several investigations and treatments are offered to couples with RPL, but most of them are not well studied. For most of these investigations and treatments, a recommendation against the intervention or treatment was formulated based on insufficient evidence. Future studies may require these recommendations to be revised.
Wider implications of the findings: The guideline provides clinicians with clear advice on best practice in RPL, based on the best evidence available. In addition, a list of research recommendations is provided to stimulate further studies in RPL. One of the most important consequences of the limited evidence is the absence of evidence for a definition of RPL.
Study funding/competing interests: The guideline was developed and funded by ESHRE, covering expenses associated with the guideline meetings, with the literature searches and with the dissemination of the guideline. The guideline group members did not receive payment. J.E. reports position funding from CARE Fertility. S.L. reports position funding from SpermComet Ltd. S.M. reports research grants, consulting and speaker's fees from GSK, BMS/Pfizer, Sanquin, Aspen, Bayer and Daiichi Sankyo. S.Q. reports speaker's fees from Ferring. The other authors report no conflicts of interest.ESHRE Pages are not externally peer reviewed. This article has been approved by the Executive Committee of ESHRE.
Keywords: ESHRE; GRADE; diagnosis; evidence based; guideline; recurrent miscarriage; recurrent pregnancy loss; treatment.
Figures
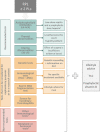
1: Including anti-HY antibodies, Natural Killer (NK) cell testing, anti-HLA antibodies.
2: Including cytokine testing/polymorphisms, assessment of polycystic ovary syndrome (PCOS), fasting insulin and fasting glucose, prolactin testing, ovarian reserve testing, luteal phase insufficiency testing, androgen testing, LHtesting, homocysteine plasma levels.
3: Low-dose aspirin and heparin are recommended after three or more pregnancy losses, or in the context of a clinical trial.
Similar articles
-
ESHRE guideline: recurrent pregnancy loss: an update in 2022.Hum Reprod Open. 2023 Mar 2;2023(1):hoad002. doi: 10.1093/hropen/hoad002. eCollection 2023. Hum Reprod Open. 2023. PMID: 36873081 Free PMC article.
-
ESHRE guideline: ovarian stimulation for IVF/ICSI†.Hum Reprod Open. 2020 May 1;2020(2):hoaa009. doi: 10.1093/hropen/hoaa009. eCollection 2020. Hum Reprod Open. 2020. PMID: 32395637 Free PMC article.
-
Evidence-based guideline: unexplained infertility†.Hum Reprod. 2023 Oct 3;38(10):1881-1890. doi: 10.1093/humrep/dead150. Hum Reprod. 2023. PMID: 37599566 Free PMC article.
-
Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome.Fertil Steril. 2018 Aug;110(3):364-379. doi: 10.1016/j.fertnstert.2018.05.004. Epub 2018 Jul 19. Fertil Steril. 2018. PMID: 30033227 Free PMC article. Review.
-
Revised guidelines for good practice in IVF laboratories (2015).Hum Reprod. 2016 Apr;31(4):685-6. doi: 10.1093/humrep/dew016. Epub 2016 Feb 17. Hum Reprod. 2016. PMID: 26908842 Review.
Cited by
-
Altered Expressions of IL-15, IFNG, and HPRT1 Genes in the Thin Endometria of Patients with Reproductive Disorders: A Prospective Comparative Study.J Clin Med. 2024 Oct 17;13(20):6184. doi: 10.3390/jcm13206184. J Clin Med. 2024. PMID: 39458137 Free PMC article.
-
Shared diagnostic genes and potential mechanisms between polycystic ovary syndrome and recurrent miscarriage revealed by integrated transcriptomics analysis and machine learning.Front Endocrinol (Lausanne). 2024 Sep 27;15:1335106. doi: 10.3389/fendo.2024.1335106. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39398336 Free PMC article.
-
Recurrent Pregnancy Loss: Immunological aetiologies and associations with mental health.Brain Behav Immun Health. 2024 Sep 19;41:100868. doi: 10.1016/j.bbih.2024.100868. eCollection 2024 Nov. Brain Behav Immun Health. 2024. PMID: 39391794 Free PMC article.
-
Estimating the costs associated with the implementation of a best practice model of care for recurrent miscarriage clinics in Ireland: a cost analysis.HRB Open Res. 2022 Nov 16;5:74. doi: 10.12688/hrbopenres.13625.1. eCollection 2022. HRB Open Res. 2022. PMID: 39359347 Free PMC article.
-
Clinical efficacy and adverse effects of LMWH combined with ASA in the treatment of RSA: A meta-analysis and systematic review.Medicine (Baltimore). 2024 Sep 13;103(37):e39603. doi: 10.1097/MD.0000000000039603. Medicine (Baltimore). 2024. PMID: 39287269 Free PMC article.
References
-
- Alonso A, Soto I, Urgelles MF, Corte JR, Rodriguez MJ, Pinto CR. Acquired and inherited thrombophilia in women with unexplained fetal losses. Am J Obstet Gynecol 2002;187:1337–1342. - PubMed
-
- Andersen AM, Andersen PK, Olsen J, Gronbaek M, Strandberg-Larsen K. Moderate alcohol intake during pregnancy and risk of fetal death. Int J Epidemiol 2012;41:405–413. - PubMed
-
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, Nasser M, Meerpohl J, Post PN, Kunz R et al. . GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–725. - PubMed
-
- Atasever M, Soyman Z, Demirel E, Gencdal S, Kelekci S. Diminished ovarian reserve: is it a neglected cause in the assessment of recurrent miscarriage? A cohort study. Fertil Steril 2016;105:1236–1240. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
