FXR Mediates Adenylyl Cyclase 8 Expression in Pancreatic β-Cells
- PMID: 31485455
- PMCID: PMC6710725
- DOI: 10.1155/2019/8915818
FXR Mediates Adenylyl Cyclase 8 Expression in Pancreatic β-Cells
Abstract
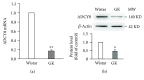
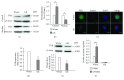
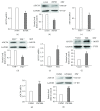
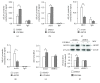
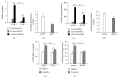
Adenylyl cyclase 8 (ADCY8) and Farnesoid X Receptor (FXR) have been identified in pancreatic β-cells and play important roles in insulin secretion. But the mechanisms underlying with respect to the regulation of ADCY8 expression in β-cells, particularly whether FXR is involved, remain unexplored. We now show that ADCY8 expression is decreased in Goto-Kakizaki (GK) rat islets compared with healthy Wistar controls. We also found that reduced ADCY8 is associated with decreased expression of FXR. Consistently, ADCY8 expression was suppressed by the knockdown of FXR in INS-1 832/13 cells, as well as the islets from FXR knockout mice. On the contrary, ADCY8 expression was increased in FXR-overexpressed INS-1 832/13 cells or in the case of FXR activation. Mechanistically, FXR directly binds to Adcy8 promoter and recruits the histone acetyltransferase Steroid Receptor Coactivator 1 (SRC1), thereby resulting in the increased acetylation of histone H3 in Adcy8 locus, promoting Adcy8 gene transcription in β-cells. Thus, this study indicates that FXR is a critical transcription factor that mediates ADCY8 expression in pancreatic β-cells and has characterized the chromatin modification associated with Adcy8 transcription.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Roux-en-Y gastric bypass enhances insulin secretion in type 2 diabetes via FXR-mediated TRPA1 expression.Mol Metab. 2019 Nov;29:1-11. doi: 10.1016/j.molmet.2019.08.009. Epub 2019 Aug 15. Mol Metab. 2019. PMID: 31668381 Free PMC article.
-
FXR/Menin-mediated epigenetic regulation of E2F3 expression controls β-cell proliferation and is increased in islets from diabetic GK rats after RYGB.Biochim Biophys Acta Mol Basis Dis. 2024 Jun;1870(5):167136. doi: 10.1016/j.bbadis.2024.167136. Epub 2024 Mar 26. Biochim Biophys Acta Mol Basis Dis. 2024. PMID: 38531483
-
Multilevel control of glucose homeostasis by adenylyl cyclase 8.Diabetologia. 2015 Apr;58(4):749-57. doi: 10.1007/s00125-014-3445-z. Epub 2014 Nov 19. Diabetologia. 2015. PMID: 25403481
-
Bile salt excretory pump: biology and pathobiology.J Pediatr Gastroenterol Nutr. 2006 Jul;43 Suppl 1:S10-6. doi: 10.1097/01.mpg.0000226385.71859.5f. J Pediatr Gastroenterol Nutr. 2006. PMID: 16819395 Review.
-
Islet gene expression and function in type 2 diabetes; studies in the Goto-Kakizaki rat and humans.Diabetes Obes Metab. 2007 Nov;9 Suppl 2:180-6. doi: 10.1111/j.1463-1326.2007.00787.x. Diabetes Obes Metab. 2007. PMID: 17919192 Review.
Cited by
-
Thiazolidinediones (TZDs) enhance insulin secretory response via GPR40 and adenylate cyclase (AC).J Cell Physiol. 2021 Dec;236(12):8137-8147. doi: 10.1002/jcp.30467. Epub 2021 Jun 16. J Cell Physiol. 2021. PMID: 34133753 Free PMC article.
-
Role of FXR in Renal Physiology and Kidney Diseases.Int J Mol Sci. 2023 Jan 26;24(3):2408. doi: 10.3390/ijms24032408. Int J Mol Sci. 2023. PMID: 36768731 Free PMC article. Review.
-
Adapting Physiology in Functional Human Islet Organogenesis.Front Cell Dev Biol. 2022 Apr 26;10:854604. doi: 10.3389/fcell.2022.854604. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35557947 Free PMC article.
-
FXR-mediated epigenetic regulation of GLP-1R expression contributes to enhanced incretin effect in diabetes after RYGB.J Cell Mol Med. 2024 Mar;28(6):e16339. doi: 10.1111/jcmm.16339. Epub 2021 Feb 21. J Cell Mol Med. 2024. PMID: 33611845 Free PMC article.
-
Physiological roles of mammalian transmembrane adenylyl cyclase isoforms.Physiol Rev. 2022 Apr 1;102(2):815-857. doi: 10.1152/physrev.00013.2021. Epub 2021 Oct 26. Physiol Rev. 2022. PMID: 34698552 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

