The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients with Oral Potentially Malignant Disorders
- PMID: 31484317
- PMCID: PMC6780631
- DOI: 10.3390/jcm8091376
The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients with Oral Potentially Malignant Disorders
Abstract
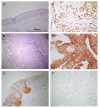
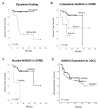
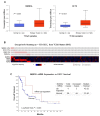
NANOG, a key regulator of pluripotency and self-renewal in embryonic and adult stem cells, is frequently overexpressed in multiple cancers, including oral squamous cell carcinoma (OSCC). It has been frequently associated with poor outcomes in epithelial cancers, and recently implicated in laryngeal tumorigenesis. On this basis, we investigated the role of NANOG protein expression as an early cancer risk biomarker in oral potentially malignant disorders (OPMD), and the impact on prognosis and disease outcomes in OSCC patients. NANOG expression was evaluated by immunohistochemistry in 55 patients with oral epithelial dysplasia, and 125 OSCC patients. Correlations with clinical and follow-up data were assessed. Nuclear NANOG expression was detected in 2 (3.6%) and cytoplasmic NANOG expression in 9 (16.4%) oral dysplasias. NANOG expression increased with the grade of dysplasia. Cytoplasmic NANOG expression and the histopathological grading were significantly correlated with oral cancer risk, although dysplasia grading was the only significant independent predictor of oral cancer development in multivariate analyses. Cytoplasmic NANOG expression was also detected in 39 (31%) OSCC samples. Positive NANOG expression was significantly associated with tobacco and alcohol consumption, and was more frequent in pN0 tumors, early I-II stages. These data unveil the clinical relevance of NANOG in early stages of OSCC tumorigenesis rather than in advanced neoplastic disease. NANOG expression emerges as an early predictor of oral cancer risk in patients with OPMD.
Keywords: NANOG; immunohistochemistry; oral cancer risk; oral epithelial dysplasia; oral squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Identification and Evaluation of Cancer Stem Cells in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia Using NANOG: An Immunohistochemical Study.Cureus. 2024 Feb 27;16(2):e55111. doi: 10.7759/cureus.55111. eCollection 2024 Feb. Cureus. 2024. PMID: 38558704 Free PMC article.
-
SOX2 Expression Is an Independent Predictor of Oral Cancer Progression.J Clin Med. 2019 Oct 21;8(10):1744. doi: 10.3390/jcm8101744. J Clin Med. 2019. PMID: 31640140 Free PMC article.
-
Elucidating the immunohistochemistry of Nanog: A transcription marker in the oral squamous cell carcinoma with emphasis on its origin as embryonic stem cell.J Oral Maxillofac Pathol. 2022 Oct-Dec;26(4):476-482. doi: 10.4103/jomfp.jomfp_347_22. Epub 2022 Dec 22. J Oral Maxillofac Pathol. 2022. PMID: 37082043 Free PMC article.
-
Molecular diagnostics in oral cancer and oral potentially malignant disorders-A clinician's guide.J Oral Pathol Med. 2020 Jan;49(1):1-8. doi: 10.1111/jop.12920. Epub 2019 Aug 3. J Oral Pathol Med. 2020. PMID: 31309636 Review.
-
Potentially malignant disorders of the oral cavity: current practice and future directions in the clinic and laboratory.Int J Cancer. 2015 Feb 1;136(3):503-15. doi: 10.1002/ijc.28754. Epub 2014 Feb 11. Int J Cancer. 2015. PMID: 24482244 Review.
Cited by
-
Evaluation of immunohistochemical expression of stem cell markers (NANOG and CD133) in normal, hyperplastic, and malignant endometrium.J Med Life. 2022 Jan;15(1):117-123. doi: 10.25122/jml-2021-0206. J Med Life. 2022. PMID: 35186145 Free PMC article.
-
Intrinsic and Extrinsic Factors Impacting Cancer Stemness and Tumor Progression.Cancers (Basel). 2022 Feb 15;14(4):970. doi: 10.3390/cancers14040970. Cancers (Basel). 2022. PMID: 35205716 Free PMC article. Review.
-
Identification and Evaluation of Cancer Stem Cells in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia Using NANOG: An Immunohistochemical Study.Cureus. 2024 Feb 27;16(2):e55111. doi: 10.7759/cureus.55111. eCollection 2024 Feb. Cureus. 2024. PMID: 38558704 Free PMC article.
-
Potential Immunohistochemical Biomarkers for Grading Oral Dysplasia: A Literature Review.Biomedicines. 2024 Mar 5;12(3):577. doi: 10.3390/biomedicines12030577. Biomedicines. 2024. PMID: 38540190 Free PMC article. Review.
-
Expression patterns and clinical significance of the potential cancer stem cell markers OCT4 and NANOG in colorectal cancer patients.Mol Cell Oncol. 2020 Jul 14;7(5):1788366. doi: 10.1080/23723556.2020.1788366. eCollection 2020. Mol Cell Oncol. 2020. PMID: 32944642 Free PMC article.
References
-
- Califano J., Westra W.H., Meininger G., Corio R., Koch W.M., Sidransky D. Genetic progression and clonal relationship of recurrent premalignant head and neck lesions. Clin. Cancer Res. 2000;6:347–352. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

