Neuronal vulnerability in Parkinson disease: Should the focus be on axons and synaptic terminals?
- PMID: 31483900
- PMCID: PMC6879792
- DOI: 10.1002/mds.27823
Neuronal vulnerability in Parkinson disease: Should the focus be on axons and synaptic terminals?
Abstract
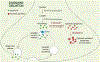
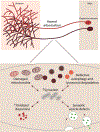
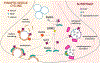
While current effective therapies are available for the symptomatic control of PD, treatments to halt the progressive neurodegeneration still do not exist. Loss of dopamine neurons in the SNc and dopamine terminals in the striatum drive the motor features of PD. Multiple lines of research point to several pathways which may contribute to dopaminergic neurodegeneration. These pathways include extensive axonal arborization, mitochondrial dysfunction, dopamine's biochemical properties, abnormal protein accumulation of α-synuclein, defective autophagy and lysosomal degradation, and synaptic impairment. Thus, understanding the essential features and mechanisms of dopaminergic neuronal vulnerability is a major scientific challenge and highlights an outstanding need for fostering effective therapies against neurodegeneration in PD. This article, which arose from the Movement Disorders 2018 Conference, discusses and reviews the possible mechanisms underlying neuronal vulnerability and potential therapeutic approaches in PD. © 2019 International Parkinson and Movement Disorder Society.
Keywords: Parkinson; dopamine; substantia nigra; synaptic; synuclein.
© 2019 International Parkinson and Movement Disorder Society.
Figures
Similar articles
-
Deficits in dopaminergic transmission precede neuron loss and dysfunction in a new Parkinson model.Proc Natl Acad Sci U S A. 2013 Oct 15;110(42):E4016-25. doi: 10.1073/pnas.1309143110. Epub 2013 Sep 30. Proc Natl Acad Sci U S A. 2013. PMID: 24082145 Free PMC article.
-
Early synaptic dysfunction induced by α-synuclein in a rat model of Parkinson's disease.Sci Rep. 2017 Jul 25;7(1):6363. doi: 10.1038/s41598-017-06724-9. Sci Rep. 2017. PMID: 28743955 Free PMC article.
-
RGS Proteins as Critical Regulators of Motor Function and Their Implications in Parkinson's Disease.Mol Pharmacol. 2020 Dec;98(6):730-738. doi: 10.1124/mol.119.118836. Epub 2020 Feb 3. Mol Pharmacol. 2020. PMID: 32015009 Free PMC article. Review.
-
Striatal synaptic bioenergetic and autophagic decline in premotor experimental parkinsonism.Brain. 2022 Jun 30;145(6):2092-2107. doi: 10.1093/brain/awac087. Brain. 2022. PMID: 35245368 Free PMC article.
-
Dopaminergic Axons: Key Recitalists in Parkinson's Disease.Neurochem Res. 2022 Feb;47(2):234-248. doi: 10.1007/s11064-021-03464-1. Epub 2021 Oct 12. Neurochem Res. 2022. PMID: 34637100 Review.
Cited by
-
Recent advances in understanding and treatment of Parkinson's disease.Fac Rev. 2020 Nov 10;9:6. doi: 10.12703/b/9-6. eCollection 2020. Fac Rev. 2020. PMID: 33659938 Free PMC article. Review.
-
Lysosomal cholesterol accumulation contributes to the movement phenotypes associated with NUS1 haploinsufficiency.Genet Med. 2021 Jul;23(7):1305-1314. doi: 10.1038/s41436-021-01137-6. Epub 2021 Mar 17. Genet Med. 2021. PMID: 33731878 Free PMC article.
-
Disentangling nigral and putaminal contribution to motor impairment and levodopa response in Parkinson's disease.NPJ Parkinsons Dis. 2022 Oct 14;8(1):132. doi: 10.1038/s41531-022-00401-z. NPJ Parkinsons Dis. 2022. PMID: 36241644 Free PMC article.
-
Linking α-synuclein-induced synaptopathy and neural network dysfunction in early Parkinson's disease.Brain Commun. 2022 Jun 22;4(4):fcac165. doi: 10.1093/braincomms/fcac165. eCollection 2022. Brain Commun. 2022. PMID: 35822101 Free PMC article. Review.
-
Interaction of Alpha Synuclein and Microtubule Organization Is Linked to Impaired Neuritic Integrity in Parkinson's Patient-Derived Neuronal Cells.Int J Mol Sci. 2022 Feb 5;23(3):1812. doi: 10.3390/ijms23031812. Int J Mol Sci. 2022. PMID: 35163733 Free PMC article.
References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature 1997;388(6645):839–40. - PubMed
-
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging 2003;24(2):197–211. - PubMed
-
- Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 1988;76(3):217–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

