GAC inhibitors with a 4-hydroxypiperidine spacer: Requirements for potency
- PMID: 31474484
- PMCID: PMC6889173
- DOI: 10.1016/j.bmcl.2019.126632
GAC inhibitors with a 4-hydroxypiperidine spacer: Requirements for potency
Abstract
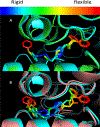
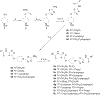
Allosteric inhibitors of glutaminase (GAC), such as BPTES, CB-839 and UPGL00019, have great promise as inhibitors of cancer cell growth, but potent inhibitors with drug-like qualities have been difficult to achieve. Here, a small library of GAC inhibitors based on the UPGL00019 core is described. This set of derivatives was designed to assess if one or both of the phenylacetyl groups flanking the UPGL00019 core can be replaced by smaller simple aliphatic acyl groups without loss in potency. We found that one of the phenylacetyl moieties can be replaced by a set of small aliphatic moieties without loss in potency. We also found that enzymatic potency co-varies with the VDW volume or the maximum projection area of the groups used as replacements of the phenylacetyl moiety and used literature X-ray data to provide an explanation for this finding.
Keywords: BPTES; CB-839; GAC; Novel glutaminase inhibitors; UPGL00019; UPGL00019 derivatives.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

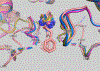
Similar articles
-
Characterization of the interactions of potent allosteric inhibitors with glutaminase C, a key enzyme in cancer cell glutamine metabolism.J Biol Chem. 2018 Mar 9;293(10):3535-3545. doi: 10.1074/jbc.M117.810101. Epub 2018 Jan 9. J Biol Chem. 2018. PMID: 29317493 Free PMC article.
-
Recent Development of Small Molecule Glutaminase Inhibitors.Curr Top Med Chem. 2018;18(6):432-443. doi: 10.2174/1568026618666180525100830. Curr Top Med Chem. 2018. PMID: 29793408 Review.
-
Design and evaluation of novel glutaminase inhibitors.Bioorg Med Chem. 2016 Apr 15;24(8):1819-39. doi: 10.1016/j.bmc.2016.03.009. Epub 2016 Mar 7. Bioorg Med Chem. 2016. PMID: 26988803 Free PMC article.
-
Conformational changes in the activation loop of mitochondrial glutaminase C: A direct fluorescence readout that distinguishes the binding of allosteric inhibitors from activators.J Biol Chem. 2017 Apr 14;292(15):6095-6107. doi: 10.1074/jbc.M116.758219. Epub 2017 Feb 14. J Biol Chem. 2017. PMID: 28196863 Free PMC article.
-
Targeting Histone Acetyltransferase MOZ/KAT6A as a New Avenue for Hematological Tumor Therapy.Curr Top Med Chem. 2020;20(5):333-335. doi: 10.2174/156802662005200304123442. Curr Top Med Chem. 2020. PMID: 32242512 Review. No abstract available.
Cited by
-
Discovery of IPN60090, a Clinical Stage Selective Glutaminase-1 (GLS-1) Inhibitor with Excellent Pharmacokinetic and Physicochemical Properties.J Med Chem. 2020 Nov 12;63(21):12957-12977. doi: 10.1021/acs.jmedchem.0c01398. Epub 2020 Oct 29. J Med Chem. 2020. PMID: 33118821 Free PMC article.
-
Targeting YTHDF1 effectively re-sensitizes cisplatin-resistant colon cancer cells by modulating GLS-mediated glutamine metabolism.Mol Ther Oncolytics. 2021 Jan 16;20:228-239. doi: 10.1016/j.omto.2021.01.001. eCollection 2021 Mar 26. Mol Ther Oncolytics. 2021. PMID: 33614908 Free PMC article.
-
Allosteric kidney-type glutaminase (GLS) inhibitors with a mercaptoethyl linker.Bioorg Med Chem. 2020 Oct 15;28(20):115698. doi: 10.1016/j.bmc.2020.115698. Epub 2020 Aug 6. Bioorg Med Chem. 2020. PMID: 33069080 Free PMC article.
References
-
- Elgadi KM; Meguid RA; Qian M; Souba WW; Abcouwer SF, Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol Genomics 1999, 1 (2), 51–62. - PubMed
-
- Szeliga M; Obara-Michlewska M, Glutamine in neoplastic cells: focus on the expression and roles of glutaminases. Neurochem Int 2009, 55 (1–3), 71–5. - PubMed
-
- Pan T; Gao L; Wu G; Shen G; Xie S; Wen H; Yang J; Zhou Y; Tu Z; Qian W, Elevated expression of glutaminase confers glucose utilization via glutaminolysis in prostate cancer. Biochem Biophys Res Commun 2015, 456 (1), 452–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Medical

