Hyper-osmolarity environment-induced oxidative stress injury promotes nucleus pulposus cell senescence in vitro
- PMID: 31471533
- PMCID: PMC6753320
- DOI: 10.1042/BSR20191711
Hyper-osmolarity environment-induced oxidative stress injury promotes nucleus pulposus cell senescence in vitro
Retraction in
-
Retraction: Hyper-osmolarity environment-induced oxidative stress injury promotes nucleus pulposus cell senescence in vitro.Biosci Rep. 2024 Aug 28;44(8):BSR-2019-1711_RET. doi: 10.1042/BSR-2019-1711_RET. Biosci Rep. 2024. PMID: 39171802 Free PMC article. No abstract available.
Abstract
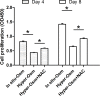
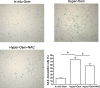
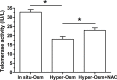
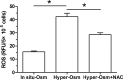
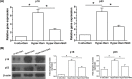
Nucleus pulposus (NP) cell senescence is involved in disc degeneration. The in situ osmolarity within the NP region is an important regulator of disc cell's biology. However, its effects on NP cell senescence remain unclear. The present study was aimed to investigate the effects and mechanism of hyper-osmolarity on NP cell senescence. Rat NP cells were cultured in the in situ-osmolarity medium and hyper-osmolarity medium. The reactive oxygen species (ROS) scavenger N-acetylcysteine (NAC) was added along with the medium to investigate the role of oxidative injury. Cell cycle, cell proliferation, senescence associated β-galactosidase (SA-β-Gal) activity, telomerase activity, expression of senescence markers (p16 and p53) and matrix molecules (aggrecan and collagen II) were tested to assess NP cell senescence. Compared with the in situ-osmolarity culture, hyper-osmolarity culture significantly decreased cell proliferation and telomerase activity, increased SA-β-Gal activity and cell fraction in the G0/G1 phase, up-regulated expression of senescence markers (p16 and p53) and down-regulated expression of matrix molecules (aggrecan and collagen II), and increased intracellular ROS accumulation. However, addition of NAC partly reversed these effects of hyper-osmolarity culture on cellular senescence and decreased ROS content in NP cells. In conclusion, a hyper-osmolarity culture promotes NP cell senescence through inducing oxidative stress injury. The present study provides new knowledge on NP cell senescence and helps us to better understand the mechanism of disc degeneration.
Keywords: intervertebral disc; nucleus pulposus; osmolarity; oxidative stress; senescence.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Resveratrol attenuates inflammation environment-induced nucleus pulposus cell senescence in vitro.Biosci Rep. 2019 May 10;39(5):BSR20190126. doi: 10.1042/BSR20190126. Print 2019 May 31. Biosci Rep. 2019. Retraction in: Biosci Rep. 2024 Aug 28;44(8):BSR-2019-0126_RET. doi: 10.1042/BSR-2019-0126_RET PMID: 30962260 Free PMC article. Retracted.
-
Acidic pH promotes nucleus pulposus cell senescence through activating the p38 MAPK pathway.Biosci Rep. 2018 Nov 13;38(6):BSR20181451. doi: 10.1042/BSR20181451. Print 2018 Dec 21. Biosci Rep. 2018. Retraction in: Biosci Rep. 2024 Aug 28;44(8):BSR-2018-1451_RET. doi: 10.1042/BSR-2018-1451_RET PMID: 30291218 Free PMC article. Retracted.
-
17beta-estradiol Attenuates TNF-α-Induced Premature Senescence of Nucleus Pulposus Cells through Regulating the ROS/NF-κB Pathway.Int J Biol Sci. 2017 Jan 15;13(2):145-156. doi: 10.7150/ijbs.16770. eCollection 2017. Int J Biol Sci. 2017. PMID: 28255267 Free PMC article.
-
N-Cadherin Attenuates High Glucose-Induced Nucleus Pulposus Cell Senescence Through Regulation of the ROS/NF-κB Pathway.Cell Physiol Biochem. 2018;47(1):257-265. doi: 10.1159/000489804. Epub 2018 May 11. Cell Physiol Biochem. 2018. PMID: 29768261
-
Nucleus pulposus cell senescence is alleviated by resveratrol through regulating the ROS/NF-κB pathway under high-magnitude compression.Biosci Rep. 2018 Jul 6;38(4):BSR20180670. doi: 10.1042/BSR20180670. Print 2018 Aug 31. Biosci Rep. 2018. Retraction in: Biosci Rep. 2024 Aug 28;44(8):BSR-2018-0670_RET. doi: 10.1042/BSR-2018-0670_RET PMID: 29875176 Free PMC article. Retracted.
Cited by
-
Senolytics: Eliminating Senescent Cells and Alleviating Intervertebral Disc Degeneration.Front Bioeng Biotechnol. 2022 Mar 2;10:823945. doi: 10.3389/fbioe.2022.823945. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35309994 Free PMC article. Review.
-
Multiscale Regulation of the Intervertebral Disc: Achievements in Experimental, In Silico, and Regenerative Research.Int J Mol Sci. 2021 Jan 12;22(2):703. doi: 10.3390/ijms22020703. Int J Mol Sci. 2021. PMID: 33445782 Free PMC article. Review.
-
Osmolar Modulation Drives Reversible Cell Cycle Exit and Human Pluripotent Cell Differentiation via NF-κВ and WNT Signaling.Adv Sci (Weinh). 2024 Feb;11(7):e2307554. doi: 10.1002/advs.202307554. Epub 2023 Dec 1. Adv Sci (Weinh). 2024. PMID: 38037844 Free PMC article.
-
Manual therapy regulates oxidative stress in aging rat lumbar intervertebral discs through the SIRT1/FOXO1 pathway.Aging (Albany NY). 2022 Mar 15;14(5):2400-2417. doi: 10.18632/aging.203949. Epub 2022 Mar 15. Aging (Albany NY). 2022. PMID: 35289767 Free PMC article.
-
Intervertebral disc cell fate during aging and degeneration: apoptosis, senescence, and autophagy.N Am Spine Soc J. 2023 Mar 11;14:100210. doi: 10.1016/j.xnsj.2023.100210. eCollection 2023 Jun. N Am Spine Soc J. 2023. PMID: 37090223 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

