Hyperglycemia-Induced Aberrant Cell Proliferation; A Metabolic Challenge Mediated by Protein O-GlcNAc Modification
- PMID: 31466420
- PMCID: PMC6769692
- DOI: 10.3390/cells8090999
Hyperglycemia-Induced Aberrant Cell Proliferation; A Metabolic Challenge Mediated by Protein O-GlcNAc Modification
Abstract
Chronic hyperglycemia has been associated with an increased prevalence of pathological conditions including cardiovascular disease, cancer, or various disorders of the immune system. In some cases, these associations may be traced back to a common underlying cause, but more often, hyperglycemia and the disturbance in metabolic balance directly facilitate pathological changes in the regular cellular functions. One such cellular function crucial for every living organism is cell cycle regulation/mitotic activity. Although metabolic challenges have long been recognized to influence cell proliferation, the direct impact of diabetes on cell cycle regulatory elements is a relatively uncharted territory. Among other "nutrient sensing" mechanisms, protein O-linked β-N-acetylglucosamine (O-GlcNAc) modification emerged in recent years as a major contributor to the deleterious effects of hyperglycemia. An increasing amount of evidence suggest that O-GlcNAc may significantly influence the cell cycle and cellular proliferation. In our present review, we summarize the current data available on the direct impact of metabolic changes caused by hyperglycemia in pathological conditions associated with cell cycle disorders. We also review published experimental evidence supporting the hypothesis that O-GlcNAc modification may be one of the missing links between metabolic regulation and cellular proliferation.
Keywords: O-GlcNAc; cancer; cell cycle; diabetes; hyperglycemia; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
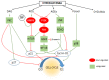
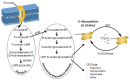
Similar articles
-
Glucose mediates the translocation of NeuroD1 by O-linked glycosylation.J Biol Chem. 2007 May 25;282(21):15589-96. doi: 10.1074/jbc.M701762200. Epub 2007 Apr 2. J Biol Chem. 2007. PMID: 17403669 Free PMC article.
-
Inhibition of ALDH2 by O-GlcNAcylation contributes to the hyperglycemic exacerbation of myocardial ischemia/reperfusion injury.Oncotarget. 2017 Mar 21;8(12):19413-19426. doi: 10.18632/oncotarget.14297. Oncotarget. 2017. PMID: 28038474 Free PMC article.
-
New insights: A role for O-GlcNAcylation in diabetic complications.Crit Rev Biochem Mol Biol. 2016 May-Jun;51(3):150-61. doi: 10.3109/10409238.2015.1135102. Epub 2016 Jan 24. Crit Rev Biochem Mol Biol. 2016. PMID: 26806492 Review.
-
Altered expression of O-GlcNAc-modified proteins in a mouse model whose glycemic status is controlled by a low carbohydrate ketogenic diet.Glycoconj J. 2013 Nov;30(8):781-9. doi: 10.1007/s10719-013-9482-x. Epub 2013 Jun 21. Glycoconj J. 2013. PMID: 23793825
-
O-Linked β-N-acetylglucosamine (O-GlcNAc) modification: a new pathway to decode pathogenesis of diabetic retinopathy.Clin Sci (Lond). 2018 Jan 19;132(2):185-198. doi: 10.1042/CS20171454. Print 2018 Jan 31. Clin Sci (Lond). 2018. PMID: 29352075 Free PMC article. Review.
Cited by
-
Disruption of O-GlcNAcylation Homeostasis Induced Ovarian Granulosa Cell Injury in Bovine.Int J Mol Sci. 2022 Jul 15;23(14):7815. doi: 10.3390/ijms23147815. Int J Mol Sci. 2022. PMID: 35887161 Free PMC article.
-
Diabetes mellitus contribution to the remodeling of the tumor microenvironment in gastric cancer.World J Gastrointest Oncol. 2021 Dec 15;13(12):1997-2012. doi: 10.4251/wjgo.v13.i12.1997. World J Gastrointest Oncol. 2021. PMID: 35070037 Free PMC article. Review.
-
High glucose macrophage exosomes enhance atherosclerosis by driving cellular proliferation & hematopoiesis.iScience. 2021 Jul 10;24(8):102847. doi: 10.1016/j.isci.2021.102847. eCollection 2021 Aug 20. iScience. 2021. PMID: 34381972 Free PMC article.
-
E3 ligase activity of Carboxyl terminus of Hsc70 interacting protein (CHIP) in Wharton's jelly derived mesenchymal stem cells improves their persistence under hyperglycemic stress and promotes the prophylactic effects against diabetic cardiac damages.Bioeng Transl Med. 2021 Jun 11;6(3):e10234. doi: 10.1002/btm2.10234. eCollection 2021 Sep. Bioeng Transl Med. 2021. PMID: 34589606 Free PMC article.
-
On the Role of Glycolysis in Early Tumorigenesis-Permissive and Executioner Effects.Cells. 2023 Apr 10;12(8):1124. doi: 10.3390/cells12081124. Cells. 2023. PMID: 37190033 Free PMC article. Review.
References
-
- World Health Organization Global Report on Diabetes. [(accessed on 11 July 2019)]; Available online: https://www.who.int/diabetes/global-report/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

