Methodological standards for the development and evaluation of clinical prediction rules: a review of the literature
- PMID: 31463368
- PMCID: PMC6704664
- DOI: 10.1186/s41512-019-0060-y
Methodological standards for the development and evaluation of clinical prediction rules: a review of the literature
Abstract
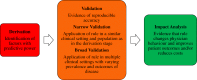
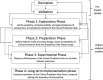
Clinical prediction rules (CPRs) that predict the absolute risk of a clinical condition or future outcome for individual patients are abundant in the medical literature; however, systematic reviews have demonstrated shortcomings in the methodological quality and reporting of prediction studies. To maximise the potential and clinical usefulness of CPRs, they must be rigorously developed and validated, and their impact on clinical practice and patient outcomes must be evaluated. This review aims to present a comprehensive overview of the stages involved in the development, validation and evaluation of CPRs, and to describe in detail the methodological standards required at each stage, illustrated with examples where appropriate. Important features of the study design, statistical analysis, modelling strategy, data collection, performance assessment, CPR presentation and reporting are discussed, in addition to other, often overlooked aspects such as the acceptability, cost-effectiveness and longer-term implementation of CPRs, and their comparison with clinical judgement. Although the development and evaluation of a robust, clinically useful CPR is anything but straightforward, adherence to the plethora of methodological standards, recommendations and frameworks at each stage will assist in the development of a rigorous CPR that has the potential to contribute usefully to clinical practice and decision-making and have a positive impact on patient care.
Keywords: Clinical prediction rule; Diagnosis; Impact studies; Implementation; Model development; Model reporting; Model validation; Prediction model; Prognosis; Risk model; Study design.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
Similar articles
-
Diagnostic clinical prediction rules for categorising low back pain: A systematic review.Musculoskeletal Care. 2023 Dec;21(4):1482-1496. doi: 10.1002/msc.1816. Epub 2023 Oct 9. Musculoskeletal Care. 2023. PMID: 37807828
-
Clinical prediction rules for children: a systematic review.Pediatrics. 2011 Sep;128(3):e666-77. doi: 10.1542/peds.2011-0043. Epub 2011 Aug 22. Pediatrics. 2011. PMID: 21859912 Review.
-
Critical appraisal of clinical prediction rules that aim to optimize treatment selection for musculoskeletal conditions.Phys Ther. 2010 Jun;90(6):843-54. doi: 10.2522/ptj.20090233. Epub 2010 Apr 22. Phys Ther. 2010. PMID: 20413577
-
Impact analysis studies of clinical prediction rules relevant to primary care: a systematic review.BMJ Open. 2016 Mar 15;6(3):e009957. doi: 10.1136/bmjopen-2015-009957. BMJ Open. 2016. PMID: 27008685 Free PMC article. Review.
-
Clinical prediction rules for prognosis and treatment prescription in neck pain: A systematic review.Musculoskelet Sci Pract. 2017 Feb;27:155-164. doi: 10.1016/j.math.2016.10.066. Epub 2016 Oct 31. Musculoskelet Sci Pract. 2017. PMID: 27852530 Review.
Cited by
-
Opioid2MME: Standardizing opioid prescriptions to morphine milligram equivalents from electronic health records.Int J Med Inform. 2022 Mar 16;162:104739. doi: 10.1016/j.ijmedinf.2022.104739. Online ahead of print. Int J Med Inform. 2022. PMID: 35325663 Free PMC article.
-
Liquid biopsies for early diagnosis of brain tumours: in silico mathematical biomarker modelling.J R Soc Interface. 2022 Aug;19(193):20220180. doi: 10.1098/rsif.2022.0180. Epub 2022 Aug 3. J R Soc Interface. 2022. PMID: 35919979 Free PMC article.
-
A deep neural network framework to derive interpretable decision rules for accurate traumatic brain injury identification of infants.BMC Med Inform Decis Mak. 2023 Apr 6;23(1):58. doi: 10.1186/s12911-023-02155-x. BMC Med Inform Decis Mak. 2023. PMID: 37024858 Free PMC article.
-
Development and Validation of a Multivariable Exercise Adherence Prediction Model for Patients with COPD: A Prospective Cohort Study.Int J Chron Obstruct Pulmon Dis. 2023 Mar 22;18:385-398. doi: 10.2147/COPD.S401023. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 36987443 Free PMC article.
-
Developing clinical prediction models when adhering to minimum sample size recommendations: The importance of quantifying bootstrap variability in tuning parameters and predictive performance.Stat Methods Med Res. 2021 Dec;30(12):2545-2561. doi: 10.1177/09622802211046388. Epub 2021 Oct 8. Stat Methods Med Res. 2021. PMID: 34623193 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources