A Membrane-Anchored Short-Peptide Fusion Inhibitor Fully Protects Target Cells from Infections of Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus
- PMID: 31462566
- PMCID: PMC6819927
- DOI: 10.1128/JVI.01177-19
A Membrane-Anchored Short-Peptide Fusion Inhibitor Fully Protects Target Cells from Infections of Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus
Abstract
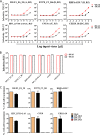
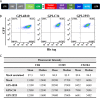
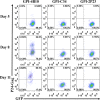
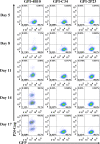
Emerging studies demonstrate that the antiviral activity of viral fusion inhibitor peptides can be dramatically improved when being chemically or genetically anchored to the cell membrane, where viral entry occurs. We previously reported that the short-peptide fusion inhibitor 2P23 and its lipid derivative possess highly potent antiviral activities against human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV). To develop a sterilizing or functional-cure strategy, here we genetically linked 2P23 and two control peptides (HIV-1 fusion inhibitor C34 and hepatitis B virus [HBV] entry inhibitor 4B10) with a glycosylphosphatidylinositol (GPI) attachment signal. As expected, GPI-anchored inhibitors were efficiently expressed on the plasma membrane of transduced TZM-bl cells and primarily directed to the lipid raft site without interfering with the expression of CD4, CCR5, and CXCR4. GPI-anchored 2P23 (GPI-2P23) completely protected TZM-bl cells from infections of divergent HIV-1, HIV-2, and SIV isolates as well as a panel of enfuvirtide (T20)-resistant mutants. GPI-2P23 also rendered the cells resistant to viral envelope-mediated cell-cell fusion and cell-associated virion-mediated cell-cell transmission. Moreover, GPI-2P23-modified human CD4+ T cells (CEMss-CCR5) fully blocked both R5- and X4-tropic HIV-1 isolates and displayed a robust survival advantage over unmodified cells during HIV-1 infection. In contrast, it was found that GPI-anchored C34 was much less effective in inhibiting HIV-2, SIV, and T20-resistant HIV-1 mutants. Therefore, our studies have demonstrated that genetically anchoring a short-peptide fusion inhibitor to the target cell membrane is a viable strategy for gene therapy of both HIV-1 and HIV-2 infections.IMPORTANCE Antiretroviral therapy with multiple drugs in combination can efficiently suppress HIV replication and dramatically reduce the morbidity and mortality associated with AIDS-related illness; however, antiretroviral therapy cannot eradiate the HIV reservoirs, and lifelong treatment is required, which often results in cumulative toxicities, drug resistance, and a multitude of complications, thus necessitating the development of sterilizing-cure or functional-cure strategies. Here, we report that genetically anchoring the short-peptide fusion inhibitor 2P23 to the cell membrane can fully prevent infections from divergent HIV-1, HIV-2, and SIV isolates as well as a panel of enfuvirtide-resistant mutants. Membrane-bound 2P23 also effectively blocks HIV-1 Env-mediated cell-cell fusion and cell-associated virion-mediated cell-cell transmission, renders CD4+ T cells nonpermissive to infection, and confers a robust survival advantage over unmodified cells. Thus, our studies verify a powerful strategy to generate resistant cells for gene therapy of both the HIV-1 and HIV-2 infections.
Keywords: HIV-1; HIV-2; fusion inhibitor; gene therapy; glycosylphosphatidylinositol.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
Cell membrane-anchored anti-HIV single-chain antibodies and bifunctional inhibitors targeting the gp41 fusion protein: new strategies for HIV gene therapy.Emerg Microbes Infect. 2022 Dec;11(1):30-49. doi: 10.1080/22221751.2021.2011616. Emerg Microbes Infect. 2022. PMID: 34821542 Free PMC article.
-
A Helical Short-Peptide Fusion Inhibitor with Highly Potent Activity against Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus.J Virol. 2016 Dec 16;91(1):e01839-16. doi: 10.1128/JVI.01839-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795437 Free PMC article.
-
Generation of HIV-resistant cells with a single-domain antibody: implications for HIV-1 gene therapy.Cell Mol Immunol. 2021 Mar;18(3):660-674. doi: 10.1038/s41423-020-00627-y. Epub 2021 Jan 18. Cell Mol Immunol. 2021. PMID: 33462383 Free PMC article.
-
Is there a future for antiviral fusion inhibitors?Curr Opin Virol. 2012 Feb;2(1):50-9. doi: 10.1016/j.coviro.2012.01.002. Epub 2012 Jan 28. Curr Opin Virol. 2012. PMID: 22440966 Review.
-
HIV-1 Entry and Membrane Fusion Inhibitors.Viruses. 2021 Apr 23;13(5):735. doi: 10.3390/v13050735. Viruses. 2021. PMID: 33922579 Free PMC article. Review.
Cited by
-
Application of CRISPR/Cas Genomic Editing Tools for HIV Therapy: Toward Precise Modifications and Multilevel Protection.Front Cell Infect Microbiol. 2022 May 25;12:880030. doi: 10.3389/fcimb.2022.880030. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35694537 Free PMC article. Review.
-
Design of a Bispecific HIV Entry Inhibitor Targeting the Cell Receptor CD4 and Viral Fusion Protein Gp41.Front Cell Infect Microbiol. 2022 May 27;12:916487. doi: 10.3389/fcimb.2022.916487. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35711654 Free PMC article.
-
Systematic Evaluation of Fluorination as Modification for Peptide-Based Fusion Inhibitors against HIV-1 Infection.Chembiochem. 2021 Dec 10;22(24):3443-3451. doi: 10.1002/cbic.202100417. Epub 2021 Oct 22. Chembiochem. 2021. PMID: 34605595 Free PMC article.
-
Screening and Molecular Modeling Evaluation of Food Peptides to Inhibit Key Targets of COVID-19 Virus.Biomolecules. 2021 Feb 22;11(2):330. doi: 10.3390/biom11020330. Biomolecules. 2021. PMID: 33671652 Free PMC article.
-
Cell membrane-anchored anti-HIV single-chain antibodies and bifunctional inhibitors targeting the gp41 fusion protein: new strategies for HIV gene therapy.Emerg Microbes Infect. 2022 Dec;11(1):30-49. doi: 10.1080/22221751.2021.2011616. Emerg Microbes Infect. 2022. PMID: 34821542 Free PMC article.
References
-
- Katlama C, Deeks SG, Autran B, Martinez-Picado J, van Lunzen J, Rouzioux C, Miller M, Vella S, Schmitz JE, Ahlers J, Richman DD, Sekaly RP. 2013. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet 381:2109–2117. doi:10.1016/S0140-6736(13)60104-X. - DOI - PMC - PubMed
-
- Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, Martinez-Picado J, Nijhuis M, Wensing AMJ, Lee H, Grant P, Nastouli E, Lambert J, Pace M, Salasc F, Monit C, Innes AJ, Muir L, Waters L, Frater J, Lever AML, Edwards SG, Gabriel IH, Olavarria E. 2019. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature 568:244–248. doi:10.1038/s41586-019-1027-4. - DOI - PMC - PubMed
-
- Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, Holmes MC, Gregory PD, Ando DG, Kalos M, Collman RG, Binder-Scholl G, Plesa G, Hwang WT, Levine BL, June CH. 2014. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370:901–910. doi:10.1056/NEJMoa1300662. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

