Reversing the Tumor Target: Establishment of a Tumor Trap
- PMID: 31456685
- PMCID: PMC6699082
- DOI: 10.3389/fphar.2019.00887
Reversing the Tumor Target: Establishment of a Tumor Trap
Abstract
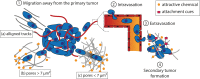
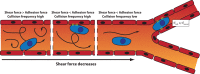
Despite the tremendous progress made in the field of cancer therapy in recent years, certain solid tumors still cannot be successfully treated. Alongside classical treatments in the form of chemotherapy and/or radiotherapy, targeted treatments such as immunotherapy that cause fewer side effects emerge as new options in the clinics. However, these alternative treatments may not be useful for treating all types of cancers, especially for killing infiltrative and circulating tumor cells (CTCs). Recent advances pursue the trapping of these cancer cells within a confined area to facilitate their removal for therapeutic and diagnostic purposes. A good understanding of the mechanisms behind tumor cell migration may drive the design of traps that mimic natural tumor niches and guide the movement of the cancer cells. To bring this trapping idea into reality, strong efforts are being made to create structured materials that imitate myelinated fibers, blood vessels, or pre-metastatic niches and incorporate chemical cues such as chemoattractants or adhesive proteins. In this review, the different strategies used (or could be used) to trap tumor cells are described, and relevant examples of their performance are analyzed.
Keywords: biomimetic trap; cancer therapy; premetastatic niche recruitment; tumor cell migration; tumor trap.
Figures


Similar articles
-
Trapping metastatic cancer cells with mechanical ratchet arrays.Acta Biomater. 2023 Oct 15;170:202-214. doi: 10.1016/j.actbio.2023.08.034. Epub 2023 Aug 23. Acta Biomater. 2023. PMID: 37619895
-
Simulation and fabrication of an integrating well-aligned silicon nanowires substrate for trapping circulating tumor cells labeled with Fe3O4 nanoparticles in a microfluidic device.Bioimpacts. 2022;12(6):533-548. doi: 10.34172/bi.2022.23393. Epub 2022 Apr 12. Bioimpacts. 2022. PMID: 36644542 Free PMC article.
-
Metformin and Docosahexaenoic Acid Hybrid Micelles for Premetastatic Niche Modulation and Tumor Metastasis Suppression.Nano Lett. 2019 Jun 12;19(6):3548-3562. doi: 10.1021/acs.nanolett.9b00495. Epub 2019 Apr 30. Nano Lett. 2019. PMID: 31026397
-
Clinical and biological significance of circulating tumor cells in cancer.Mol Oncol. 2016 Mar;10(3):408-17. doi: 10.1016/j.molonc.2016.01.010. Epub 2016 Feb 10. Mol Oncol. 2016. PMID: 26899533 Free PMC article. Review.
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
Cited by
-
The matrix-dependent 3D spheroid model of the migration of non-small cell lung cancer: a step towards a rapid automated screening.Front Mol Biosci. 2021 Mar 25;8:610407. doi: 10.3389/fmolb.2021.610407. eCollection 2021. Front Mol Biosci. 2021. PMID: 34422897 Free PMC article.
-
Hyaluronic Acid Scaffolds for Loco-Regional Therapy in Nervous System Related Disorders.Int J Mol Sci. 2022 Oct 12;23(20):12174. doi: 10.3390/ijms232012174. Int J Mol Sci. 2022. PMID: 36293030 Free PMC article. Review.
-
Mesenchymal Stromal-Like Cells in the Glioma Microenvironment: What Are These Cells?Cancers (Basel). 2020 Sep 15;12(9):2628. doi: 10.3390/cancers12092628. Cancers (Basel). 2020. PMID: 32942567 Free PMC article. Review.
-
Development of a Synthetic, Injectable Hydrogel to Capture Residual Glioblastoma and Glioblastoma Stem-Like Cells with CXCL12-Mediated Chemotaxis.Adv Healthc Mater. 2023 Jun;12(14):e2300671. doi: 10.1002/adhm.202300671. Epub 2023 Apr 20. Adv Healthc Mater. 2023. PMID: 37014179 Free PMC article.
-
Drug Delivery Systems in the Development of Novel Strategies for Glioblastoma Treatment.Pharmaceutics. 2022 Jun 1;14(6):1189. doi: 10.3390/pharmaceutics14061189. Pharmaceutics. 2022. PMID: 35745762 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

