Promastigote secretory gel from natural and unnatural sand fly vectors exacerbate Leishmania major and Leishmania tropica cutaneous leishmaniasis in mice
- PMID: 31452467
- PMCID: PMC6939171
- DOI: 10.1017/S0031182019001069
Promastigote secretory gel from natural and unnatural sand fly vectors exacerbate Leishmania major and Leishmania tropica cutaneous leishmaniasis in mice
Abstract
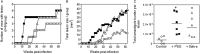
Leishmania rely heavily on glycans to complete their digenetic life cycle in both mammalian and phlebotomine sand fly hosts. Leishmania promastigotes secrete a proteophosphoglycan-rich gel (Promastigote Secretory Gel, PSG) that is regurgitated during transmission and can exacerbate infection in the skin. Here we explored the role of PSG from natural Leishmania-sand fly vector combinations by obtaining PSG from Leishmania (L.) major-infected Phlebotomus (P.) papatasi and P. duboscqi and L. tropica-infected P. arabicus. We found that, in addition to the vector's saliva, the PSG from L. major and L. tropica potently exacerbated cutaneous infection in BALB/c mice, improved the probability of developing a patent cutaneous lesion, parasite growth and the evolution of the lesion. Of note, the presence of PSG in the inoculum more than halved the prepatent period of cutaneous L. tropica infection from an average of 32 weeks to 13 weeks. In addition, L. major and L. tropica PSG extracted from the permissive experimental vector, Lutzomyia (Lu.) longipalpis, also exacerbated infections in mice. These results reinforce and extend the hypothesis that PSG is an important and evolutionarily conserved component of Leishmania infection that can be used to facilitate experimental infection for drug and vaccine screening.
Keywords: Cutaneous leishmaniasis; Leishmania; Leishmania major; Leishmania mexicana; Leishmania tropica; PSG; sand fly; transmission; zoonoses.
Figures
Similar articles
-
Proteophosophoglycans regurgitated by Leishmania-infected sand flies target the L-arginine metabolism of host macrophages to promote parasite survival.PLoS Pathog. 2009 Aug;5(8):e1000555. doi: 10.1371/journal.ppat.1000555. Epub 2009 Aug 21. PLoS Pathog. 2009. PMID: 19696894 Free PMC article.
-
Leishmania proteophosphoglycans regurgitated from infected sand flies accelerate dermal wound repair and exacerbate leishmaniasis via insulin-like growth factor 1-dependent signalling.PLoS Pathog. 2018 Jan 19;14(1):e1006794. doi: 10.1371/journal.ppat.1006794. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29352310 Free PMC article.
-
Leishmania infantum proteophosphoglycans regurgitated by the bite of its natural sand fly vector, Lutzomyia longipalpis, promote parasite establishment in mouse skin and skin-distant tissues.Microbes Infect. 2010 Oct;12(11):875-9. doi: 10.1016/j.micinf.2010.05.014. Epub 2010 Jun 16. Microbes Infect. 2010. PMID: 20561596
-
Leishmania tropica (Kinetoplastida: Trypanosomatidae)--a perplexing parasite.Folia Parasitol (Praha). 2003 Dec;50(4):241-50. doi: 10.14411/fp.2003.042. Folia Parasitol (Praha). 2003. PMID: 14971592 Review.
-
Transmission patterns of Leishmania tropica around the Mediterranean basin: Could Morocco be impacted by a zoonotic spillover?PLoS Negl Trop Dis. 2022 Jan 13;16(1):e0010009. doi: 10.1371/journal.pntd.0010009. eCollection 2022 Jan. PLoS Negl Trop Dis. 2022. PMID: 35025884 Free PMC article. Review.
Cited by
-
Characterization of a new Leishmania major strain for use in a controlled human infection model.Nat Commun. 2021 Jan 11;12(1):215. doi: 10.1038/s41467-020-20569-3. Nat Commun. 2021. PMID: 33431825 Free PMC article.
-
FVB/NJ strain as a mouse model for cutaneous leishmaniasis by Leishmania (L.) amazonensis.Mem Inst Oswaldo Cruz. 2024 Mar 15;119:e230182. doi: 10.1590/0074-02760230182. eCollection 2024. Mem Inst Oswaldo Cruz. 2024. PMID: 38511814 Free PMC article.
-
Role of Virulence Factors of Trypanosomatids in the Insect Vector and Putative Genetic Events Involved in Surface Protein Diversity.Front Cell Infect Microbiol. 2022 Apr 28;12:807172. doi: 10.3389/fcimb.2022.807172. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35573777 Free PMC article. Review.
-
Insights into Leishmania Molecules and Their Potential Contribution to the Virulence of the Parasite.Vet Sci. 2021 Feb 20;8(2):33. doi: 10.3390/vetsci8020033. Vet Sci. 2021. PMID: 33672776 Free PMC article. Review.
References
-
- Anderson CF, Lira R, Kamhawi S, Belkaid Y, Wynn TA and Sacks D (2008) IL-10 and TGF-beta control the establishment of persistent and transmissible infections produced by Leishmania tropica in C57BL/6 mice. Journal of Immunology 180, 4090–4097. - PubMed
-
- Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J and Sacks DL (1998) Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva pre-exposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. Journal of Experimental Medicine 188, 1941–1953. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

