Serum response factor (SRF) promotes ROS generation and hepatic stellate cell activation by epigenetically stimulating NCF1/2 transcription
- PMID: 31442911
- PMCID: PMC6831835
- DOI: 10.1016/j.redox.2019.101302
Serum response factor (SRF) promotes ROS generation and hepatic stellate cell activation by epigenetically stimulating NCF1/2 transcription
Abstract
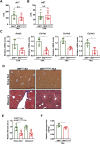
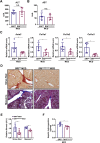
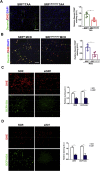
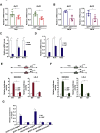
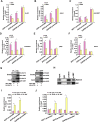
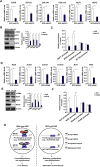
Activation of hepatic stellate cells (HSC) is a hallmark event in liver fibrosis. Accumulation of reactive oxygen species (ROS) serves as a driving force for HSC activation. The regulatory subunits of the NOX complex, NCF1 (p47phox) and NCF2 (p67phox), are up-regulated during HSC activation contributing to ROS production and liver fibrosis. The transcriptional mechanism underlying NCF1/2 up-regulation is not clear. In the present study we investigated the role of serum response factor (SRF) in HSC activation focusing on the transcriptional regulation of NCF1/2. We report that compared to wild type littermates HSC-conditional SRF knockout (CKO) mice exhibited a mortified phenotype of liver fibrosis induced by thioacetamide (TAA) injection or feeding with a methionine-and-choline deficient diet (MCD). More importantly, SRF deletion attenuated ROS levels in HSCs in vivo. Similarly, SRF knockdown in cultured HSCs suppressed ROS production in vitro. Further analysis revealed that SRF deficiency resulted in repression of NCF1/NCF2 expression. Mechanistically, SRF regulated epigenetic transcriptional activation of NCF1/NCF2 by interacting with and recruiting the histone acetyltransferase KAT8 during HSC activation. In conclusion, we propose that SRF integrates transcriptional activation of NCF1/NCF2 and ROS production to promote liver fibrosis.
Keywords: Hepatic stellate cell; Liver fibrosis; Neutrophil cytosolic factor; Reactive oxygen species; Serum response factor; Transcriptional regulation.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Ablation of serum response factor in hepatic stellate cells attenuates liver fibrosis.J Mol Med (Berl). 2019 Nov;97(11):1521-1533. doi: 10.1007/s00109-019-01831-8. Epub 2019 Aug 21. J Mol Med (Berl). 2019. PMID: 31435710
-
Serum response factor activates peroxidasin transcription to block senescence of hepatic stellate cells.Life Sci. 2023 Sep 1;328:121824. doi: 10.1016/j.lfs.2023.121824. Epub 2023 Jun 1. Life Sci. 2023. PMID: 37270170
-
Deficiency of NOX1 or NOX4 Prevents Liver Inflammation and Fibrosis in Mice through Inhibition of Hepatic Stellate Cell Activation.PLoS One. 2015 Jul 29;10(7):e0129743. doi: 10.1371/journal.pone.0129743. eCollection 2015. PLoS One. 2015. PMID: 26222337 Free PMC article.
-
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and liver fibrosis: A review.Cell Biochem Funct. 2018 Aug;36(6):292-302. doi: 10.1002/cbf.3351. Epub 2018 Jul 20. Cell Biochem Funct. 2018. PMID: 30028028 Review.
-
Role of NADPH oxidases in the redox biology of liver fibrosis.Redox Biol. 2015 Dec;6:106-111. doi: 10.1016/j.redox.2015.07.005. Epub 2015 Jul 14. Redox Biol. 2015. PMID: 26204504 Free PMC article. Review.
Cited by
-
Transcriptional Activation of Matricellular Protein Spondin2 (SPON2) by BRG1 in Vascular Endothelial Cells Promotes Macrophage Chemotaxis.Front Cell Dev Biol. 2020 Aug 14;8:794. doi: 10.3389/fcell.2020.00794. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32974343 Free PMC article.
-
Inhibiting IRE1α-endonuclease activity decreases tumor burden in a mouse model for hepatocellular carcinoma.Elife. 2020 Oct 26;9:e55865. doi: 10.7554/eLife.55865. Elife. 2020. PMID: 33103995 Free PMC article.
-
Activation of Galectin-3 (LGALS3) Transcription by Injurious Stimuli in the Liver Is Commonly Mediated by BRG1.Front Cell Dev Biol. 2019 Nov 26;7:310. doi: 10.3389/fcell.2019.00310. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31850346 Free PMC article.
-
A GSK3-SRF Axis Mediates Angiotensin II Induced Endothelin Transcription in Vascular Endothelial Cells.Front Cell Dev Biol. 2021 Jul 26;9:698254. doi: 10.3389/fcell.2021.698254. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34381779 Free PMC article.
-
Redox-sensitive activation of CCL7 by BRG1 in hepatocytes during liver injury.Redox Biol. 2021 Oct;46:102079. doi: 10.1016/j.redox.2021.102079. Epub 2021 Jul 24. Redox Biol. 2021. PMID: 34454163 Free PMC article.
References
-
- Leto T.L., Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxidants Redox Signal. 2006;8:1549–1561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

