Combination PD-1 and PD-L1 Blockade Promotes Durable Neoantigen-Specific T Cell-Mediated Immunity in Pancreatic Ductal Adenocarcinoma
- PMID: 31433988
- PMCID: PMC7975822
- DOI: 10.1016/j.celrep.2019.07.059
Combination PD-1 and PD-L1 Blockade Promotes Durable Neoantigen-Specific T Cell-Mediated Immunity in Pancreatic Ductal Adenocarcinoma
Abstract
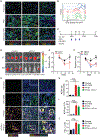
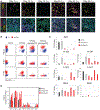
Pancreatic ductal adenocarcinoma (PDA) is a lethal cancer resistant to immunotherapy. We create a PDA mouse model and show that neoantigen expression is required for intratumoral T cell accumulation and response to immune checkpoint blockade. By generating a peptide:MHC tetramer, we identify that PDA induces rapid intratumoral, and progressive systemic, tumor-specific T cell exhaustion. Monotherapy PD-1 or PD-L1 blockade enhances systemic T cell expansion and induces objective responses that require systemic T cells. However, tumor escape variants defective in IFNγ-inducible Tap1 and MHC class I cell surface expression ultimately emerge. Combination PD-1 + PD-L1 blockade synergizes therapeutically by increasing intratumoral KLRG1+Lag3-TNFα+ tumor-specific T cells and generating memory T cells capable of expanding to spontaneous tumor recurrence, thereby prolonging animal survival. Our studies support that PD-1 and PD-L1 are relevant immune checkpoints in PDA and identify a combination for clinical testing in those patients with neoantigen-specific T cells.
Keywords: PD-1; PD-L1; PDA; T cells; acquired resistance; immunotherapy; neoepitope; pancreatic cancer.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures





Similar articles
-
PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors.J Immunother. 2015 Jan;38(1):1-11. doi: 10.1097/CJI.0000000000000062. J Immunother. 2015. PMID: 25415283 Free PMC article.
-
IFN-γ Promotes Epithelial-Mesenchymal Transition and the Expression of PD-L1 in Pancreatic Cancer.J Surg Res. 2019 Aug;240:115-123. doi: 10.1016/j.jss.2019.02.038. Epub 2019 Mar 27. J Surg Res. 2019. PMID: 30927618
-
PAK1 inhibition increases TRIM21-induced PD-L1 degradation and enhances responses to anti-PD-1 therapy in pancreatic cancer.Biochim Biophys Acta Mol Basis Dis. 2024 Aug;1870(6):167236. doi: 10.1016/j.bbadis.2024.167236. Epub 2024 May 11. Biochim Biophys Acta Mol Basis Dis. 2024. PMID: 38740225
-
PD-1/PD-L1 and immunotherapy for pancreatic cancer.Cancer Lett. 2017 Oct 28;407:57-65. doi: 10.1016/j.canlet.2017.08.006. Epub 2017 Aug 18. Cancer Lett. 2017. PMID: 28826722 Review.
-
PD-1/PD-L1 Blockade Therapy for Tumors with Downregulated MHC Class I Expression.Int J Mol Sci. 2017 Jun 21;18(6):1331. doi: 10.3390/ijms18061331. Int J Mol Sci. 2017. PMID: 28635644 Free PMC article. Review.
Cited by
-
A bispecific anti-PD-1 and PD-L1 antibody induces PD-1 cleavage and provides enhanced anti-tumor activity.Oncoimmunology. 2024 Feb 16;13(1):2316945. doi: 10.1080/2162402X.2024.2316945. eCollection 2024. Oncoimmunology. 2024. PMID: 38379869 Free PMC article.
-
Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation.Front Immunol. 2021 Mar 9;12:636568. doi: 10.3389/fimmu.2021.636568. eCollection 2021. Front Immunol. 2021. PMID: 33767702 Free PMC article. Review.
-
Deep Learning-Based Classification of Epithelial-Mesenchymal Transition for Predicting Response to Therapy in Clear Cell Renal Cell Carcinoma.Front Oncol. 2022 Jan 24;11:782515. doi: 10.3389/fonc.2021.782515. eCollection 2021. Front Oncol. 2022. PMID: 35141144 Free PMC article.
-
Comparison of programmed death ligand 1 immunostaining for pancreatic ductal adenocarcinoma between paired cytological and surgical samples.Cytojournal. 2021 Nov 3;18:28. doi: 10.25259/Cytojournal_78_2020. eCollection 2021. Cytojournal. 2021. PMID: 34876919 Free PMC article.
-
T-Cell Receptor Repertoire Sequencing and Its Applications: Focus on Infectious Diseases and Cancer.Int J Mol Sci. 2022 Aug 2;23(15):8590. doi: 10.3390/ijms23158590. Int J Mol Sci. 2022. PMID: 35955721 Free PMC article. Review.
References
-
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, and Davis MM (1996). Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96. - PubMed
-
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, et al.; Australian Pancreatic Cancer Genome Initiative (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52. - PubMed
-
- Balachandran VP, quksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, et al.; Australian Pancreatic Cancer Genome Initiative; Garvan Institute of Medical Research; Prince of Wales Hospital; Royal North Shore Hospital; University of Glasgow; St Vincent’s Hospital; QIMR Berghofer Medical Research Institute; University of Melbourne, Centre for Cancer Research; University of Queensland, Institute for Molecular Bioscience; Bankstown Hospital; Liverpool Hospital; Royal Prince Alfred Hospital, Chris O’Brien Lifehouse; Westmead Hospital; Fremantle Hospital; St John of God Healthcare; Royal Adelaide Hospital; Flinders Medical Centre; Envoi Pathology; Princess Alexandria Hospital; Austin Hospital; Johns Hopkins Medical Institutes; ARC-Net Centre for Applied Research on Cancer (2017). Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516. - PMC - PubMed
-
- Bellone G, Turletti A, Artusio E, Mareschi K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G, and Rodeck U (1999). Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am. J. Pathol 155, 537–547. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

