Recent advances in microfluidic methods in cancer liquid biopsy
- PMID: 31431816
- PMCID: PMC6697033
- DOI: 10.1063/1.5087690
Recent advances in microfluidic methods in cancer liquid biopsy
Abstract
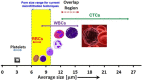
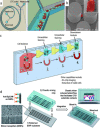
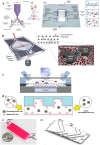
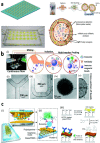
Early cancer detection, its monitoring, and therapeutical prediction are highly valuable, though extremely challenging targets in oncology. Significant progress has been made recently, resulting in a group of devices and techniques that are now capable of successfully detecting, interpreting, and monitoring cancer biomarkers in body fluids. Precise information about malignancies can be obtained from liquid biopsies by isolating and analyzing circulating tumor cells (CTCs) or nucleic acids, tumor-derived vesicles or proteins, and metabolites. The current work provides a general overview of the latest on-chip technological developments for cancer liquid biopsy. Current challenges for their translation and their application in various clinical settings are discussed. Microfluidic solutions for each set of biomarkers are compared, and a global overview of the major trends and ongoing research challenges is given. A detailed analysis of the microfluidic isolation of CTCs with recent efforts that aimed at increasing purity and capture efficiency is provided as well. Although CTCs have been the focus of a vast microfluidic research effort as the key element for obtaining relevant information, important clinical insights can also be achieved from alternative biomarkers, such as classical protein biomarkers, exosomes, or circulating-free nucleic acids. Finally, while most work has been devoted to the analysis of blood-based biomarkers, we highlight the less explored potential of urine as an ideal source of molecular cancer biomarkers for point-of-care lab-on-chip devices.
Figures

Similar articles
-
Latest advances and perspectives of liquid biopsy for cancer diagnostics driven by microfluidic on-chip assays.Lab Chip. 2023 Jun 28;23(13):2922-2941. doi: 10.1039/d2lc00837h. Lab Chip. 2023. PMID: 37291937 Review.
-
Emerging Lab-on-a-Chip Approaches for Liquid Biopsy in Lung Cancer: Status in CTCs and ctDNA Research and Clinical Validation.Cancers (Basel). 2021 Apr 27;13(9):2101. doi: 10.3390/cancers13092101. Cancers (Basel). 2021. PMID: 33925308 Free PMC article. Review.
-
Sensor-Integrated Microfluidic Approaches for Liquid Biopsies Applications in Early Detection of Cancer.Sensors (Basel). 2020 Feb 28;20(5):1317. doi: 10.3390/s20051317. Sensors (Basel). 2020. PMID: 32121271 Free PMC article. Review.
-
Recent advances in integrated microfluidics for liquid biopsies and future directions.Biosens Bioelectron. 2022 Dec 1;217:114715. doi: 10.1016/j.bios.2022.114715. Epub 2022 Sep 14. Biosens Bioelectron. 2022. PMID: 36174359 Review.
-
Microfluidic Chip-Based Cancer Diagnosis and Prediction of Relapse by Detecting Circulating Tumor Cells and Circulating Cancer Stem Cells.Cancers (Basel). 2021 Mar 18;13(6):1385. doi: 10.3390/cancers13061385. Cancers (Basel). 2021. PMID: 33803846 Free PMC article. Review.
Cited by
-
Procoagulant tumor microvesicles attach to endothelial cells on biochips under microfluidic flow.Biomicrofluidics. 2019 Dec 6;13(6):064124. doi: 10.1063/1.5123462. eCollection 2019 Nov. Biomicrofluidics. 2019. PMID: 31832122 Free PMC article.
-
High‑throughput and continuous flow isolation of rare circulating tumor cells and clusters in gastric cancer from human whole blood samples using electromagnetic vibration‑based filtration.Oncol Rep. 2020 Jun;43(6):1975-1985. doi: 10.3892/or.2020.7567. Epub 2020 Mar 30. Oncol Rep. 2020. PMID: 32236590 Free PMC article.
-
Immuno-digital invasive cleavage assay for analyzing Alzheimer's amyloid ß-bound extracellular vesicles.Alzheimers Res Ther. 2022 Oct 3;14(1):140. doi: 10.1186/s13195-022-01073-w. Alzheimers Res Ther. 2022. PMID: 36184615 Free PMC article.
-
Current and future applications of biomarkers in samples collected through minimally invasive methods for cancer medicine and population-based research.Am J Hum Biol. 2022 Nov;34(11):e23665. doi: 10.1002/ajhb.23665. Epub 2021 Aug 9. Am J Hum Biol. 2022. PMID: 34374148 Free PMC article. Review.
-
Visualization and Measurements of Blood Cells Flowing in Microfluidic Systems and Blood Rheology: A Personalized Medicine Perspective.J Pers Med. 2020 Nov 26;10(4):249. doi: 10.3390/jpm10040249. J Pers Med. 2020. PMID: 33256123 Free PMC article. Review.
References
-
- World Health Organization, International Agency for Research on Cancer (2018).
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
