Optimisation and validation of hydrogel-based brain tissue clearing shows uniform expansion across anatomical regions and spatial scales
- PMID: 31427619
- PMCID: PMC6700094
- DOI: 10.1038/s41598-019-48460-2
Optimisation and validation of hydrogel-based brain tissue clearing shows uniform expansion across anatomical regions and spatial scales
Abstract
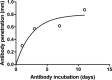
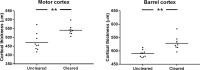
Imaging of fixed tissue is routine in experimental neuroscience, but is limited by the depth of tissue that can be imaged using conventional methods. Optical clearing of brain tissue using hydrogel-based methods (e.g. CLARITY) allows imaging of large volumes of tissue and is rapidly becoming commonplace in the field. However, these methods suffer from a lack of standardized protocols and validation of the effect they have upon tissue morphology. We present a simple and reliable protocol for tissue clearing along with a quantitative assessment of the effect of tissue clearing upon morphology. Tissue clearing caused tissue swelling (compared to conventional methods), but this swelling was shown to be similar across spatial scales and the variation was within limits acceptable to the field. The results of many studies rely upon an assumption of uniformity in tissue swelling, and by demonstrating this quantitatively, research using these methods can be interpreted more reliably.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Simple and Rapid Tissue Clearing Method for Three-Dimensional Histology of the Pancreas.Curr Protoc Cell Biol. 2017 Dec 11;77:19.20.1-19.20.10. doi: 10.1002/cpcb.34. Curr Protoc Cell Biol. 2017. PMID: 29227554
-
ScaleS: an optical clearing palette for biological imaging.Nat Neurosci. 2015 Oct;18(10):1518-29. doi: 10.1038/nn.4107. Epub 2015 Sep 14. Nat Neurosci. 2015. PMID: 26368944
-
Rationalisation and Validation of an Acrylamide-Free Procedure in Three-Dimensional Histological Imaging.PLoS One. 2016 Jun 30;11(6):e0158628. doi: 10.1371/journal.pone.0158628. eCollection 2016. PLoS One. 2016. PMID: 27359336 Free PMC article.
-
Advances and perspectives in tissue clearing using CLARITY.J Chem Neuroanat. 2017 Dec;86:19-34. doi: 10.1016/j.jchemneu.2017.07.005. Epub 2017 Jul 17. J Chem Neuroanat. 2017. PMID: 28728966 Review.
-
Current Status of Tissue Clearing and the Path Forward in Neuroscience.ACS Chem Neurosci. 2021 Jan 6;12(1):5-29. doi: 10.1021/acschemneuro.0c00563. Epub 2020 Dec 16. ACS Chem Neurosci. 2021. PMID: 33326739 Review.
References
-
- Spalteholz, W. Über das Durchsichtigmachen von menschlichen und tierischen Präparaten (Leipzig: Hirzel, 1914).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

