The Dry Eye Assessment and Management (DREAM) extension study - A randomized clinical trial of withdrawal of supplementation with omega-3 fatty acid in patients with dry eye disease
- PMID: 31425752
- PMCID: PMC7004875
- DOI: 10.1016/j.jtos.2019.08.002
The Dry Eye Assessment and Management (DREAM) extension study - A randomized clinical trial of withdrawal of supplementation with omega-3 fatty acid in patients with dry eye disease
Abstract
Purpose: To determine effects of continued or discontinued use of omega-3 (ω3) fatty acid supplements through a randomized withdrawal trial among patients assigned to ω3 supplements in the first year of the DREAM study.
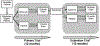
Methods: Patients who were initially assigned to ω3 (3000 mg) for 12 months in the primary trial were randomized 1:1 to ω3 active supplements or placebos (refined olive oil) for 12 more months. The primary outcome was change in the Ocular Surface Disease Index (OSDI) score. Secondary outcomes included change in conjunctival staining, corneal staining, tear break-up time, Schirmer test, and adverse events.
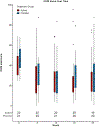
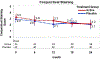
Results: Among 22 patients assigned to ω3 and 21 to placebo supplements, the mean change in OSDI score between month 12 and 24 was similar between treatment groups (mean difference in change -0.6 points, 95% confidence interval [CI], (-10.7, 9.5), p = 0.91). There were no significant differences between groups in mean change in conjunctival staining (difference in mean change -0.5 points; 95% CI (-1.2, 0.3)), corneal staining (-0.3 points; 95% CI (-1.2, 0.3)), tear break-up time (-0.8 s; 95% CI (-2.6, 0.9)) and Schirmer test (0.6 mm, 95% CI (-2.0, 3.2)). Rates of adverse events were similar in both groups.
Conclusion: Among patients who received ω3 supplements for 12 months in the primary trial, those discontinuing use of ω3 for an additional 12 months did not have significantly worse outcomes compared to those who continued use of ω3. ClinicalTrials.gov number NCT02128763.
Keywords: Dry eye disease; Omega-3 fatty acids; Randomized clinical trial.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease.N Engl J Med. 2018 May 3;378(18):1681-1690. doi: 10.1056/NEJMoa1709691. Epub 2018 Apr 13. N Engl J Med. 2018. PMID: 29652551 Free PMC article. Clinical Trial.
-
Effect of Omega-3 Fatty Acids Dietary Supplementation on Ocular Surface and Tear Film in Diabetic Patients with Dry Eye.J Am Coll Nutr. 2017 Jan;36(1):38-43. doi: 10.1080/07315724.2016.1170643. Epub 2016 Oct 31. J Am Coll Nutr. 2017. PMID: 27797641
-
A Randomized, Double-Masked, Placebo-Controlled Clinical Trial of Two Forms of Omega-3 Supplements for Treating Dry Eye Disease.Ophthalmology. 2017 Jan;124(1):43-52. doi: 10.1016/j.ophtha.2016.09.023. Epub 2016 Nov 3. Ophthalmology. 2017. PMID: 27817918 Clinical Trial.
-
Punctal occlusion for dry eye syndrome.Cochrane Database Syst Rev. 2017 Jun 26;6(6):CD006775. doi: 10.1002/14651858.CD006775.pub3. Cochrane Database Syst Rev. 2017. PMID: 28649802 Free PMC article. Review.
-
Omegas and Dry Eye: More Knowledge, More Questions.Optom Vis Sci. 2015 Sep;92(9):948-56. doi: 10.1097/OPX.0000000000000655. Optom Vis Sci. 2015. PMID: 26164311 Review.
Cited by
-
Polymorphisms in Lymphotoxin-Alpha as the "Missing Link" in Prognosticating Favourable Response to Omega-3 Supplementation for Dry Eye Disease: A Narrative Review.Int J Mol Sci. 2023 Feb 20;24(4):4236. doi: 10.3390/ijms24044236. Int J Mol Sci. 2023. PMID: 36835647 Free PMC article. Review.
-
A comparative review of evaporative dry eye disease and meibomian gland dysfunction in dogs and humans.Vet Ophthalmol. 2023 Apr;26 Suppl 1(Suppl 1):16-30. doi: 10.1111/vop.13066. Epub 2023 Feb 14. Vet Ophthalmol. 2023. PMID: 36786010 Free PMC article. Review.
-
Placebo administration for dry eye disease: a level I evidence based systematic review and meta-analysis.Int J Clin Pharm. 2022 Oct;44(5):1087-1101. doi: 10.1007/s11096-022-01439-y. Epub 2022 Aug 8. Int J Clin Pharm. 2022. PMID: 35939178 Free PMC article. Review.
-
Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease.Cochrane Database Syst Rev. 2019 Dec 18;12(12):CD011016. doi: 10.1002/14651858.CD011016.pub2. Cochrane Database Syst Rev. 2019. PMID: 31847055 Free PMC article.
-
Dry Eye Disease: Early Recognition with Guidance on Management and Treatment for Primary Care Family Physicians.Ophthalmol Ther. 2020 Dec;9(4):877-888. doi: 10.1007/s40123-020-00308-z. Epub 2020 Oct 22. Ophthalmol Ther. 2020. PMID: 33090327 Free PMC article. Review.
References
-
- Pflugfelder SC, Geerling G, Kinoshita S, Lemp MA, McCulley J, Nelson D, et al. Management and therapy of dry eye disease: report of the management and therapy subcommittee of the International Dry Eye WorkShop (2007). Ocular Surf 2007;5:163–78. - PubMed
-
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf 2017;15:276–83. - PubMed
-
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocul Surf 2017;15:334–65. - PubMed

