Induction and Kinetics of Complement-Fixing Antibodies Against Plasmodium vivax Merozoite Surface Protein 3α and Relationship With Immunoglobulin G Subclasses and Immunoglobulin M
- PMID: 31419296
- PMCID: PMC6834073
- DOI: 10.1093/infdis/jiz407
Induction and Kinetics of Complement-Fixing Antibodies Against Plasmodium vivax Merozoite Surface Protein 3α and Relationship With Immunoglobulin G Subclasses and Immunoglobulin M
Abstract
Background: Complement-fixing antibodies are important mediators of protection against Plasmodium falciparum malaria. However, complement-fixing antibodies remain uncharacterized for Plasmodium vivax malaria. P. vivax merozoite surface protein 3α (PvMSP3α) is a target of acquired immunity and a potential vaccine candidate.
Methods: Plasma from children and adults with P. vivax malaria in Sabah, Malaysia, were collected during acute infection, 7 and 28 days after drug treatment. Complement-fixing antibodies and immunoglobulin M and G (IgM and IgG), targeting 3 distinctive regions of PvMSP3α, were measured by means of enzyme-linked immunosorbent assay.
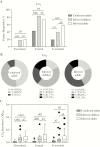
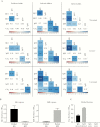
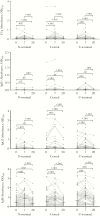
Results: The seroprevalence of complement-fixing antibodies was highest against the PvMSP3α central region (77.6%). IgG1, IgG3, and IgM were significantly correlated with C1q fixation, and both purified IgG and IgM were capable of mediating C1q fixation to PvMSP3α. Complement-fixing antibody levels were similar between age groups, but IgM was predominant in children and IgG3 more prevalent in adults. Levels of functional antibodies increased after acute infection through 7 days after treatment but rapidly waned by day 28.
Conclusion: Our study demonstrates that PvMSP3α antibodies acquired during P. vivax infection can mediate complement fixation and shows the important influence of age in shaping these specific antibody responses. Further studies are warranted to understand the role of these functional antibodies in protective immunity against P. vivax malaria.
Keywords: Plasmodium vivax; Complement-fixing antibodies; PvMSP3α; malaria.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Naturally acquired immune responses to P. vivax merozoite surface protein 3α and merozoite surface protein 9 are associated with reduced risk of P. vivax malaria in young Papua New Guinean children.PLoS Negl Trop Dis. 2013 Nov 14;7(11):e2498. doi: 10.1371/journal.pntd.0002498. eCollection 2013 Nov. PLoS Negl Trop Dis. 2013. PMID: 24244763 Free PMC article.
-
Anti-Plasmodium vivax merozoite surface protein 3 ϒ (PvMSP3 ϒ) antibodies upon natural infection.Sci Rep. 2024 Apr 26;14(1):9595. doi: 10.1038/s41598-024-59153-w. Sci Rep. 2024. PMID: 38671033 Free PMC article.
-
Immunoglobulin GM 3 23 5,13,14 phenotype is strongly associated with IgG1 antibody responses to Plasmodium vivax vaccine candidate antigens PvMSP1-19 and PvAMA-1.Malar J. 2010 Aug 9;9:229. doi: 10.1186/1475-2875-9-229. Malar J. 2010. PMID: 20696056 Free PMC article.
-
Immunological markers of Plasmodium vivax exposure and immunity: a systematic review and meta-analysis.BMC Med. 2014 Sep 9;12:150. doi: 10.1186/s12916-014-0150-1. BMC Med. 2014. PMID: 25199532 Free PMC article. Review.
-
Development and longevity of naturally acquired antibody and memory B cell responses against Plasmodium vivax infection.PLoS Negl Trop Dis. 2024 Oct 24;18(10):e0012600. doi: 10.1371/journal.pntd.0012600. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39446698 Free PMC article. Review.
Cited by
-
Plasmodium falciparum-specific IgM B cells dominate in children, expand with malaria, and produce functional IgM.J Exp Med. 2021 Apr 5;218(4):e20200901. doi: 10.1084/jem.20200901. J Exp Med. 2021. PMID: 33661303 Free PMC article.
-
Antibody-mediated complement activation in pathology and protection.Immunol Cell Biol. 2020 Apr;98(4):305-317. doi: 10.1111/imcb.12324. Epub 2020 Apr 6. Immunol Cell Biol. 2020. PMID: 32142167 Free PMC article. Review.
-
Lessons Learned for Pathogenesis, Immunology, and Disease of Erythrocytic Parasites: Plasmodium and Babesia.Front Cell Infect Microbiol. 2021 Aug 3;11:685239. doi: 10.3389/fcimb.2021.685239. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34414129 Free PMC article. Review.
-
Plasmodium vivax vaccine: What is the best way to go?Front Immunol. 2023 Jan 16;13:910236. doi: 10.3389/fimmu.2022.910236. eCollection 2022. Front Immunol. 2023. PMID: 36726991 Free PMC article. Review.
-
Immunological characterization of a VIR protein family member (VIR-14) in Plasmodium vivax-infected subjects from different epidemiological regions in Africa and South America.PLoS Negl Trop Dis. 2023 Apr 7;17(4):e0011229. doi: 10.1371/journal.pntd.0011229. eCollection 2023 Apr. PLoS Negl Trop Dis. 2023. PMID: 37027391 Free PMC article.
References
-
- Drakeley CJ, Bousema JT, Akim NI, et al. . Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol 2006; 28:185–90. - PubMed

