KPNA3 Confers Sorafenib Resistance to Advanced Hepatocellular Carcinoma via TWIST Regulated Epithelial-Mesenchymal Transition
- PMID: 31417635
- PMCID: PMC6692625
- DOI: 10.7150/jca.31448
KPNA3 Confers Sorafenib Resistance to Advanced Hepatocellular Carcinoma via TWIST Regulated Epithelial-Mesenchymal Transition
Abstract
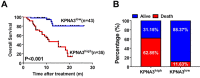
Sorafenib, a multikinase inhibitor, is a new standard treatment for patients with advanced hepatocellular carcinoma (HCC). However, resistance to this regimen is frequently observed in clinical practice, and the molecular basis of this resistance remains largely unknown. Herein, the antitumor activity of sorafenib was assessed in 16 patient-derived xenograft (PDX) models of HCC. Gene expression analysis was conducted to identify factors that promote sorafenib resistance. Quantitative RT-PCR and immunoblotting were used to determine gene expression and activation of signaling pathways. Cell proliferation, clone formation, and transwell assays were conducted to evaluate drug-sensitivity, proliferation, and invasiveness, respectively. Kaplan-Meier analysis was used to evaluate the predictive power of biomarkers for sorafenib response. Differential gene expression analysis suggested that sorafenib resistance correlated with high karyopherin subunit alpha 3 (KPNA3) expression. Overexpression of KPNA3 in HCC cells enhanced tumor cell growth and invasiveness. Interestingly, KPNA3 was found to trigger epithelial-mesenchymal transition (EMT), a key process mediating drug resistance. On a mechanistic level, KPNA3 increased phosphorylation of AKT, which then phosphorylated ERK, and ultimately promoted TWIST expression to induce EMT and sorafenib resistance. Moreover, retrospective analysis revealed that HCC patients with low KPNA3 expression had remarkably longer survival after sorafenib treatment. Finally, we have identified a novel KPNA3-AKT-ERK-TWIST signaling cascade that promotes EMT and mediates sorafenib resistance in HCC. These findings suggest that KPNA3 is a promising biomarker for predicting patient responsiveness to sorafenib. Targeting KPNA3 may also contribute to resolving sorafenib resistance in HCC.
Keywords: drug resistance; epithelial-mesenchymal transition; hepatocellular carcinoma; patient-derived xenograft; personalized medicine.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells.PLoS One. 2017 Sep 21;12(9):e0185088. doi: 10.1371/journal.pone.0185088. eCollection 2017. PLoS One. 2017. PMID: 28934275 Free PMC article.
-
Increased expression of SLC46A3 to oppose the progression of hepatocellular carcinoma and its effect on sorafenib therapy.Biomed Pharmacother. 2019 Jun;114:108864. doi: 10.1016/j.biopha.2019.108864. Epub 2019 Apr 10. Biomed Pharmacother. 2019. PMID: 30981107
-
LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR-128/CD151 pathway in hepatocellular carcinoma.J Cell Physiol. 2019 Mar;234(3):2788-2794. doi: 10.1002/jcp.27095. Epub 2018 Aug 21. J Cell Physiol. 2019. PMID: 30132868
-
Epithelial-to-Mesenchymal Transition: A Mediator of Sorafenib Resistance in Advanced Hepatocellular Carcinoma.Curr Cancer Drug Targets. 2017;17(8):698-706. doi: 10.2174/1568009617666170427104356. Curr Cancer Drug Targets. 2017. PMID: 28460616 Review.
-
The current status of tumor microenvironment and cancer stem cells in sorafenib resistance of hepatocellular carcinoma.Front Oncol. 2023 Jul 27;13:1204513. doi: 10.3389/fonc.2023.1204513. eCollection 2023. Front Oncol. 2023. PMID: 37576900 Free PMC article. Review.
Cited by
-
Molecular Bases of Drug Resistance in Hepatocellular Carcinoma.Cancers (Basel). 2020 Jun 23;12(6):1663. doi: 10.3390/cancers12061663. Cancers (Basel). 2020. PMID: 32585893 Free PMC article. Review.
-
The Bright and the Dark Side of TGF-β Signaling in Hepatocellular Carcinoma: Mechanisms, Dysregulation, and Therapeutic Implications.Cancers (Basel). 2022 Feb 14;14(4):940. doi: 10.3390/cancers14040940. Cancers (Basel). 2022. PMID: 35205692 Free PMC article. Review.
-
ZIC2 promotes cancer stem cell traits via up-regulating OCT4 expression in lung adenocarcinoma cells.J Cancer. 2020 Aug 19;11(20):6070-6080. doi: 10.7150/jca.44367. eCollection 2020. J Cancer. 2020. PMID: 32922547 Free PMC article.
-
Clinical Evolution of Epithelial-Mesenchymal Transition in Human Carcinomas.Cancer Res. 2020 Jan 15;80(2):304-318. doi: 10.1158/0008-5472.CAN-18-3539. Epub 2019 Nov 15. Cancer Res. 2020. PMID: 31732654 Free PMC article.
-
Unique genetic signature and selection footprints in Dutch population of German Longhaired Pointer dogs.Anim Genet. 2022 Dec;53(6):829-840. doi: 10.1111/age.13253. Epub 2022 Aug 22. Anim Genet. 2022. PMID: 35993291 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. - PubMed
-
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835–53. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF. et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

