Infralimbic Estradiol Enhances Neuronal Excitability and Facilitates Extinction of Cocaine Seeking in Female Rats via a BDNF/TrkB Mechanism
- PMID: 31417375
- PMCID: PMC6684748
- DOI: 10.3389/fnbeh.2019.00168
Infralimbic Estradiol Enhances Neuronal Excitability and Facilitates Extinction of Cocaine Seeking in Female Rats via a BDNF/TrkB Mechanism
Abstract
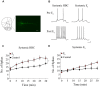
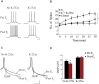
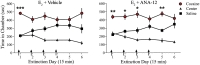
Women are more susceptible to developing cocaine dependence than men, but paradoxically, are more responsive to treatment. The potent estrogen, 17β-estradiol (E2), mediates these effects by augmenting cocaine seeking but also promoting extinction of cocaine seeking through E2's memory-enhancing functions. Although we have previously shown that E2 facilitates extinction, the neuroanatomical locus of action and underlying mechanisms are unknown. Here we demonstrate that E2 infused directly into the infralimbic-medial prefrontal cortex (IL-mPFC), a region critical for extinction consolidation, enhances extinction of cocaine seeking in ovariectomized (OVX) female rats. Using patch-clamp electrophysiology, we show that E2 may facilitate extinction by potentiating intrinsic excitability of IL-mPFC neurons. Because the mnemonic effects of E2 are known to be regulated by brain-derived neurotrophic factor (BDNF) and its receptor, tropomyosin-related kinase B (TrkB), we examined whether BDNF/TrkB signaling was necessary for E2-induced enhancement of excitability and extinction. We found that E2-mediated increases in excitability of IL-mPFC neurons were abolished by Trk receptor blockade. Moreover, blockade of TrkB signaling impaired E2-facilitated extinction of cocaine seeking in OVX female rats. Thus, E2 enhances IL-mPFC neuronal excitability in a TrkB-dependent manner to support extinction of cocaine seeking. Our findings suggest that pharmacological enhancement of E2 or BDNF/TrkB signaling during extinction-based therapies would improve therapeutic outcome in cocaine-addicted women.
Keywords: brain-derived neurotrophic factor; cocaine abuse; conditioned place preference (CPP); electrophysiology; estrogens; extinction learning; intrinsic excitability; medial prefrontal cortex (mPFC).
Figures

Similar articles
-
Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference.J Neurosci. 2014 Apr 23;34(17):6057-64. doi: 10.1523/JNEUROSCI.4980-13.2014. J Neurosci. 2014. PMID: 24760865 Free PMC article.
-
Vagus nerve stimulation (VNS) modulates synaptic plasticity in the rat infralimbic cortex via Trk-B receptor activation to reduce drug-seeking.bioRxiv [Preprint]. 2024 Jan 26:2024.01.25.577293. doi: 10.1101/2024.01.25.577293. bioRxiv. 2024. Update in: J Neurosci. 2024 Jun 5;44(23):e0107242024. doi: 10.1523/JNEUROSCI.0107-24.2024. PMID: 38328140 Free PMC article. Updated. Preprint.
-
BDNF/TrkB signaling mediates the anorectic action of estradiol in the nucleus tractus solitarius.Oncotarget. 2017 Sep 19;8(48):84028-84038. doi: 10.18632/oncotarget.21062. eCollection 2017 Oct 13. Oncotarget. 2017. PMID: 29137402 Free PMC article.
-
Prefrontal Neuronal Excitability Maintains Cocaine-Associated Memory During Retrieval.Front Behav Neurosci. 2018 Jun 14;12:119. doi: 10.3389/fnbeh.2018.00119. eCollection 2018. Front Behav Neurosci. 2018. PMID: 29962941 Free PMC article.
-
Effects of prenatal exposure to cocaine on brain structure and function.Prog Brain Res. 2014;211:277-89. doi: 10.1016/B978-0-444-63425-2.00012-X. Prog Brain Res. 2014. PMID: 24968785 Review.
Cited by
-
Infralimbic Cortex Biases Preference Decision Making for Offspring over Competing Cocaine-Associated Stimuli in New Mother Rats.eNeuro. 2020 Jul 13;7(4):ENEURO.0460-19.2020. doi: 10.1523/ENEURO.0460-19.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32631896 Free PMC article.
-
17β-estradiol activation of dorsal hippocampal TrkB is independent of increased mature BDNF expression and is required for enhanced memory consolidation in female mice.Psychoneuroendocrinology. 2021 Mar;125:105110. doi: 10.1016/j.psyneuen.2020.105110. Epub 2020 Dec 15. Psychoneuroendocrinology. 2021. PMID: 33352471 Free PMC article.
-
17β-Estradiol Attenuates Neuropathic Pain Caused by Spared Nerve Injury by Upregulating CIC-3 in the Dorsal Root Ganglion of Ovariectomized Rats.Front Neurosci. 2019 Nov 8;13:1205. doi: 10.3389/fnins.2019.01205. eCollection 2019. Front Neurosci. 2019. PMID: 31787875 Free PMC article.
-
Distinct neuronal excitability alterations of medial prefrontal cortex in early-life neglect model of rats.Animal Model Exp Med. 2022 Jun;5(3):274-280. doi: 10.1002/ame2.12252. Epub 2022 Jun 23. Animal Model Exp Med. 2022. PMID: 35748035 Free PMC article.
-
Estradiol effects on spatial memory in women.Behav Brain Res. 2022 Jan 24;417:113592. doi: 10.1016/j.bbr.2021.113592. Epub 2021 Sep 22. Behav Brain Res. 2022. PMID: 34560131 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

