Evaluation of α-synuclein and apolipoprotein E as potential biomarkers in cerebrospinal fluid to monitor pharmacotherapeutic efficacy in dopamine dictated disease states of Parkinson's disease and schizophrenia
- PMID: 31410011
- PMCID: PMC6650621
- DOI: 10.2147/NDT.S205550
Evaluation of α-synuclein and apolipoprotein E as potential biomarkers in cerebrospinal fluid to monitor pharmacotherapeutic efficacy in dopamine dictated disease states of Parkinson's disease and schizophrenia
Erratum in
-
Erratum: Evaluation of α-Synuclein and Apolipoprotein E as Potential Biomarkers in Cerebrospinal Fluid to Monitor Pharmacotherapeutic Efficacy in Dopamine Dictated Disease States of Parkinson's Disease and Schizophrenia [Corrigendum].Neuropsychiatr Dis Treat. 2022 Jun 27;18:1271-1272. doi: 10.2147/NDT.S379262. eCollection 2022. Neuropsychiatr Dis Treat. 2022. PMID: 35784514 Free PMC article.
Abstract
Background and objective: Dopamine plays an important role in the disease pathology of Parkinson's disease and schizophrenia. These two neuropsychiatric disorders represent disease end points of the dopaminergic spectrum where Parkinson's disease represents dopamine deficit and schizophrenia represents dopamine hyperactivity in the mid-brain. Therefore, current treatment strategies aim to restore normal dopamine levels. However, during treatment patients develop adverse effects due to overshooting of physiological levels of dopamine leading to psychosis in Parkinson's disease, and extrapyramidal symptoms in schizophrenia. Absence of any laboratory tests hampers modulation of pharmacotherapy. Apolipoprotein E and α-synuclein have an important role in the neuropathology of these two diseases. The objective of this study was to evaluate cerebrospinal fluid (CSF) concentrations of apolipoprotein E and α-synuclein in patients with these two diseases so that they may serve as biomarkers to monitor therapy in Parkinson's disease and schizophrenia.
Methods: Drug-naïve Parkinson's disease patients and Parkinson's disease patients treated with dopaminergic therapy, neurological controls, schizophrenic patients treated with antidopaminergic therapy, and drug-naïve schizophrenic patients were recruited for the study and CSF was collected. Enzyme-linked immunosorbent assays were carried out to estimate the concentrations of apolipoprotein E and α-synuclein. Pathway analysis was done to establish a possible role of these two proteins in various pathways in these two dopamine dictated diseases.
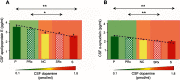
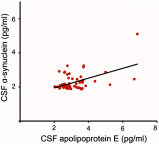
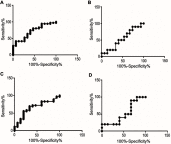
Results: Apolipoprotein E and α-synuclein CSF concentrations have an inverse correlation along the entire dopaminergic clinical spectrum. Pathway analysis convincingly establishes a plausible hypothesis for their co-regulation in the pathogenesis of Parkinson's disease and schizophrenia. Each protein by itself or as a combination has encouraging sensitivity and specificity values of more than 55%.
Conclusion: The dynamic variation of these two proteins along the spectrum is ideal for them to be pursued as pharmacotherapeutic biomarkers in CSF to monitor pharmacological efficacy in Parkinson's disease and schizophrenia.
Keywords: Parkinson’s disease; apolipoprotein E; biomarkers; cerebrospinal fluid; dopamine; schizophrenia; treatment monitoring; α-synuclein.
Conflict of interest statement
The authors report no conflicts of interest in regard to this work.
Figures


Similar articles
-
Cerebrospinal Fluid Proteomics For Identification Of α2-Macroglobulin As A Potential Biomarker To Monitor Pharmacological Therapeutic Efficacy In Dopamine Dictated Disease States Of Parkinson's Disease And Schizophrenia.Neuropsychiatr Dis Treat. 2019 Oct 2;15:2853-2867. doi: 10.2147/NDT.S214217. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 31632033 Free PMC article.
-
Evaluation of Serum Apolipoprotein E as a Potential Biomarker for Pharmacological Therapeutic Efficacy Monitoring in Dopamine Dictated Disease Spectrum of Schizophrenia and Parkinson's disease: A Preliminary Study.J Cent Nerv Syst Dis. 2018 Oct 9;10:1179573518803585. doi: 10.1177/1179573518803585. eCollection 2018. J Cent Nerv Syst Dis. 2018. PMID: 30327579 Free PMC article.
-
2D-DIGE as a strategy to identify serum protein biomarkers to monitor pharmacological efficacy in dopamine-dictated states of Parkinson's disease and schizophrenia.Neuropsychiatr Dis Treat. 2019 Apr 24;15:1031-1044. doi: 10.2147/NDT.S198559. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 31114209 Free PMC article.
-
Biological confounders for the values of cerebrospinal fluid proteins in Parkinson's disease and related disorders.J Neurochem. 2016 Oct;139 Suppl 1:290-317. doi: 10.1111/jnc.13390. Epub 2016 Feb 10. J Neurochem. 2016. PMID: 26452984 Review.
-
Interactions of dopamine, iron, and alpha-synuclein linked to dopaminergic neuron vulnerability in Parkinson's disease and Neurodegeneration with Brain Iron Accumulation disorders.Neurobiol Dis. 2022 Dec;175:105920. doi: 10.1016/j.nbd.2022.105920. Epub 2022 Nov 7. Neurobiol Dis. 2022. PMID: 36351559 Review.
Cited by
-
Cerebrospinal Fluid Proteomics For Identification Of α2-Macroglobulin As A Potential Biomarker To Monitor Pharmacological Therapeutic Efficacy In Dopamine Dictated Disease States Of Parkinson's Disease And Schizophrenia.Neuropsychiatr Dis Treat. 2019 Oct 2;15:2853-2867. doi: 10.2147/NDT.S214217. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 31632033 Free PMC article.
-
Identification of potential biomarkers to predict organ morbidity in COVID-19: A repository based proteomics perspective.Biochem Biophys Rep. 2023 Sep;35:101493. doi: 10.1016/j.bbrep.2023.101493. Epub 2023 Jun 2. Biochem Biophys Rep. 2023. PMID: 37304132 Free PMC article.
-
Altered Sphingolipid Hydrolase Activities and Alpha-Synuclein Level in Late-Onset Schizophrenia.Metabolites. 2023 Dec 31;14(1):30. doi: 10.3390/metabo14010030. Metabolites. 2023. PMID: 38248833 Free PMC article.
-
Alpha Synuclein Toxicity and Non-Motor Parkinson's.Cells. 2024 Jul 27;13(15):1265. doi: 10.3390/cells13151265. Cells. 2024. PMID: 39120295 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

