A Protein Antagonist of Activation-Induced Cytidine Deaminase Encoded by a Complex Mouse Retrovirus
- PMID: 31409681
- PMCID: PMC6692512
- DOI: 10.1128/mBio.01678-19
A Protein Antagonist of Activation-Induced Cytidine Deaminase Encoded by a Complex Mouse Retrovirus
Abstract
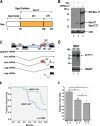
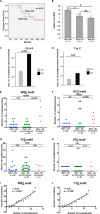
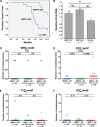
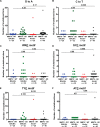
Complex human-pathogenic retroviruses cause high morbidity and mortality worldwide, but resist antiviral drugs and vaccine development due to evasion of the immune response. A complex retrovirus, mouse mammary tumor virus (MMTV), requires replication in B and T lymphocytes for mammary gland transmission and is antagonized by the innate immune restriction factor murine Apobec3 (mA3). To determine whether the regulatory/accessory protein Rem affects innate responses to MMTV, a splice-donor mutant (MMTV-SD) lacking Rem expression was injected into BALB/c mice. Mammary tumors induced by MMTV-SD had a lower proviral load, lower incidence, and longer latency than mammary tumors induced by wild-type MMTV (MMTV-WT). MMTV-SD proviruses had many G-to-A mutations on the proviral plus strand, but also C-to-T transitions within WRC motifs. Similarly, a lymphomagenic MMTV variant lacking Rem expression showed decreased proviral loads and increased WRC motif mutations relative to those in wild-type-virus-induced tumors, consistent with activation-induced cytidine deaminase (AID) mutagenesis in lymphoid cells. These mutations are typical of the Apobec family member AID, a B-cell-specific mutagenic protein involved in antibody variable region hypermutation. In contrast, mutations in WRC motifs and proviral loads were similar in MMTV-WT and MMTV-SD proviruses from tumors in AID-insufficient mice. AID was not packaged in MMTV virions. Rem coexpression in transfection experiments led to AID proteasomal degradation. Our data suggest that rem specifies a human-pathogenic immunodeficiency virus type 1 (HIV-1) Vif-like protein that inhibits AID and antagonizes innate immunity during MMTV replication in lymphocytes.IMPORTANCE Complex retroviruses, such as human-pathogenic immunodeficiency virus type 1 (HIV-1), cause many human deaths. These retroviruses produce lifelong infections through viral proteins that interfere with host immunity. The complex retrovirus mouse mammary tumor virus (MMTV) allows for studies of host-pathogen interactions not possible in humans. A mutation preventing expression of the MMTV Rem protein in two different MMTV strains decreased proviral loads in tumors and increased viral genome mutations typical of an evolutionarily ancient enzyme, AID. Although the presence of AID generally improves antibody-based immunity, it may contribute to human cancer progression. We observed that coexpression of MMTV Rem and AID led to AID destruction. Our results suggest that Rem is the first known protein inhibitor of AID and that further experiments could lead to new disease treatments.
Keywords: AID inhibitor; Apobec3; activation-induced cytidine deaminase; mouse mammary tumor virus; retroviruses.
Copyright © 2019 Singh et al.
Figures



Similar articles
-
Mouse APOBEC3 Restriction of Retroviruses.Viruses. 2020 Oct 27;12(11):1217. doi: 10.3390/v12111217. Viruses. 2020. PMID: 33121095 Free PMC article. Review.
-
Apobec-mediated retroviral hypermutation in vivo is dependent on mouse strain.PLoS Pathog. 2024 Aug 29;20(8):e1012505. doi: 10.1371/journal.ppat.1012505. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39208378 Free PMC article.
-
APOBEC3 inhibition of mouse mammary tumor virus infection: the role of cytidine deamination versus inhibition of reverse transcription.J Virol. 2013 May;87(9):4808-17. doi: 10.1128/JVI.00112-13. Epub 2013 Feb 28. J Virol. 2013. PMID: 23449789 Free PMC article.
-
Apobec-Mediated Retroviral Hypermutation In Vivo is Dependent on Mouse Strain.bioRxiv [Preprint]. 2023 Nov 2:2023.11.02.565355. doi: 10.1101/2023.11.02.565355. bioRxiv. 2023. Update in: PLoS Pathog. 2024 Aug 29;20(8):e1012505. doi: 10.1371/journal.ppat.1012505. PMID: 37961113 Free PMC article. Updated. Preprint.
-
MMTV-induced mutations in mouse mammary tumors: their potential relevance to human breast cancer.Breast Cancer Res Treat. 1996;39(1):33-44. doi: 10.1007/BF01806076. Breast Cancer Res Treat. 1996. PMID: 8738604 Review.
Cited by
-
Mouse APOBEC3 Restriction of Retroviruses.Viruses. 2020 Oct 27;12(11):1217. doi: 10.3390/v12111217. Viruses. 2020. PMID: 33121095 Free PMC article. Review.
-
APOBECs: Our fickle friends?PLoS Pathog. 2023 May 18;19(5):e1011364. doi: 10.1371/journal.ppat.1011364. eCollection 2023 May. PLoS Pathog. 2023. PMID: 37200235 Free PMC article. No abstract available.
-
Unconventional p97/VCP-Mediated Endoplasmic Reticulum-to-Endosome Trafficking of a Retroviral Protein.J Virol. 2021 Jun 24;95(14):e0053121. doi: 10.1128/JVI.00531-21. Epub 2021 Jun 24. J Virol. 2021. PMID: 33952644 Free PMC article.
-
Apobec-mediated retroviral hypermutation in vivo is dependent on mouse strain.PLoS Pathog. 2024 Aug 29;20(8):e1012505. doi: 10.1371/journal.ppat.1012505. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39208378 Free PMC article.
-
A Retrotranslocation Assay That Predicts Defective VCP/p97-Mediated Trafficking of a Retroviral Signal Peptide.mBio. 2022 Feb 22;13(1):e0295321. doi: 10.1128/mBio.02953-21. Epub 2022 Jan 4. mBio. 2022. PMID: 35089078 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
