PD-1 of Sigmodon hispidus: Gene identification, characterization and preliminary evaluation of expression in inactivated RSV vaccine-induced enhanced respiratory disease
- PMID: 31406266
- PMCID: PMC6690999
- DOI: 10.1038/s41598-019-48225-x
PD-1 of Sigmodon hispidus: Gene identification, characterization and preliminary evaluation of expression in inactivated RSV vaccine-induced enhanced respiratory disease
Abstract
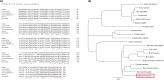
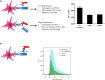
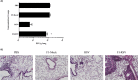
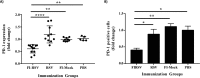
Sigmodon hispidus or cotton rat is an excellent animal model for studying human infections of respiratory viruses including respiratory syncytial virus (RSV), which is the leading cause of hospitalization in infants and causes high rates of infection in the elderly and immunocompromised patient populations. Despite several decades of research, no vaccine has been licensed whereas inactivated vaccines have been shown to induce severe adverse reaction in a clinical trial, with other forms of RSV vaccine also found to induce enhanced disease in preclinical animal studies. While arguably the cotton rat is the best small animal model for evaluation of RSV vaccines and antivirals, many important genes of the immune system remain to be isolated. Programmed cell death-1 (PD-1) plays an integral role in regulating many aspects of immunity by inducing suppressive signals. In this study, we report the isolation of mRNA encoding the cotton rat PD-1 (crPD-1) and characterization of the PD-1 protein. crPD-1 bound to its cognate ligand on dendritic cells and effectively suppressed cytokine secretion. Moreover, using the newly acquired gene sequence, we observed a decreased level of crPD-1 levels in cotton rats with enhanced respiratory disease induced by inactivated RSV vaccine, unraveling a new facet of vaccine-induced disease.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The cotton rat Sigmodon hispidus model of respiratory syncytial virus infection.Curr Top Microbiol Immunol. 2013;372:347-58. doi: 10.1007/978-3-642-38919-1_17. Curr Top Microbiol Immunol. 2013. PMID: 24362698
-
A Single-Dose Recombinant Parainfluenza Virus 5-Vectored Vaccine Expressing Respiratory Syncytial Virus (RSV) F or G Protein Protected Cotton Rats and African Green Monkeys from RSV Challenge.J Virol. 2017 May 12;91(11):e00066-17. doi: 10.1128/JVI.00066-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28298602 Free PMC article.
-
Unveiling Integrated Functional Pathways Leading to Enhanced Respiratory Disease Associated With Inactivated Respiratory Syncytial Viral Vaccine.Front Immunol. 2019 Mar 29;10:597. doi: 10.3389/fimmu.2019.00597. eCollection 2019. Front Immunol. 2019. PMID: 30984178 Free PMC article.
-
Immunopathogenesis associated with formaldehyde-inactivated RSV vaccine in preclinical and clinical studies.Expert Rev Vaccines. 2017 Apr;16(4):351-360. doi: 10.1080/14760584.2017.1260452. Epub 2016 Nov 21. Expert Rev Vaccines. 2017. PMID: 27841687 Review.
-
Advances in and the potential of vaccines for respiratory syncytial virus.Expert Rev Respir Med. 2013 Aug;7(4):411-27. doi: 10.1586/17476348.2013.814409. Expert Rev Respir Med. 2013. PMID: 23964629 Review.
References
-
- Jin H-T, Ahmed R, Okazaki T. Role of PD-1 in Regulating T-Cell. Immunity. 2010;350:17–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

