Role of Asparagine Endopeptidase in Mediating Wild-Type p53 Inactivation of Glioblastoma
- PMID: 31400201
- PMCID: PMC7156939
- DOI: 10.1093/jnci/djz155
Role of Asparagine Endopeptidase in Mediating Wild-Type p53 Inactivation of Glioblastoma
Abstract
Background: Isocitrate dehydrogenase wild-type (WT) glioblastoma (GBM) accounts for 90% of all GBMs, yet only 27% of isocitrate dehydrogenase WT-GBMs have p53 mutations. However, the tumor surveillance function of WT-p53 in GBM is subverted by mechanisms that are not fully understood.
Methods: We investigated the proteolytic inactivation of WT-p53 by asparaginyl endopeptidase (AEP) and its effects on GBM progression in cancer cells, murine models, and patients' specimens using biochemical and functional assays. The sera of healthy donors (n = 48) and GBM patients (n = 20) were examined by enzyme-linked immunosorbent assay. Furthermore, effects of AEP inhibitors on GBM progression were evaluated in murine models (n = 6-8 per group). The statistical significance between groups was determined using two-tailed Student t tests.
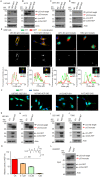
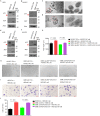
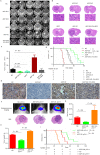
Results: We demonstrate that AEP binds to and directly cleaves WT-p53, resulting in the inhibition of WT-p53-mediated tumor suppressor function in both tumor cells and stromal cells via extracellular vesicle communication. High expression of uncleavable p53-N311A-mutant rescue AEP-induced tumorigenesis, proliferation, and anti-apoptotic abilities. Knock down or pharmacological inhibition of AEP reduced tumorigenesis and prolonged survival in murine models. However, overexpression of AEP promoted tumorigenesis and shortened the survival time. Moreover, high AEP levels in GBM tissues were associated with a poor prognosis of GBM patients (n = 83; hazard ratio = 3.94, 95% confidence interval = 1.87 to 8.28; P < .001). A correlation was found between high plasma AEP levels and a larger tumor size in GBM patients (r = 0.6, P = .03), which decreased dramatically after surgery.
Conclusions: Our results indicate that AEP promotes GBM progression via inactivation of WT-p53 and may serve as a prognostic and therapeutic target for GBM.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures





Comment in
-
Inactivation of Wild-Type p53 by Asparagine Endopeptidase in Glioblastoma: An Opportunity to Target the "Undruggable".J Natl Cancer Inst. 2020 Apr 1;112(4):327-329. doi: 10.1093/jnci/djz156. J Natl Cancer Inst. 2020. PMID: 31400198 Free PMC article. No abstract available.
Similar articles
-
Functional role of asparaginyl endopeptidase ubiquitination by TRAF6 in tumor invasion and metastasis.J Natl Cancer Inst. 2014 Apr;106(4):dju012. doi: 10.1093/jnci/dju012. Epub 2014 Mar 7. J Natl Cancer Inst. 2014. PMID: 24610907
-
Detection of p53 mutations in proliferating vascular cells in glioblastoma multiforme.J Neurosurg. 2015 Feb;122(2):317-23. doi: 10.3171/2014.10.JNS132159. Epub 2014 Nov 21. J Neurosurg. 2015. PMID: 25415071
-
Expression of alternatively spliced mdm2 transcripts correlates with stabilized wild-type p53 protein in human glioblastoma cells.Int J Cancer. 1999 Mar 15;80(6):930-4. doi: 10.1002/(sici)1097-0215(19990315)80:6<930::aid-ijc20>3.0.co;2-m. Int J Cancer. 1999. PMID: 10074928
-
The Mechanism of Asparagine Endopeptidase in the Progression of Malignant Tumors: A Review.Cells. 2021 May 10;10(5):1153. doi: 10.3390/cells10051153. Cells. 2021. PMID: 34068767 Free PMC article. Review.
-
Asparagine endopeptidase is an innovative therapeutic target for neurodegenerative diseases.Expert Opin Ther Targets. 2016 Oct;20(10):1237-45. doi: 10.1080/14728222.2016.1182990. Epub 2016 May 13. Expert Opin Ther Targets. 2016. PMID: 27115710 Free PMC article. Review.
Cited by
-
Midkine promotes glioblastoma progression via PI3K-Akt signaling.Cancer Cell Int. 2021 Sep 23;21(1):509. doi: 10.1186/s12935-021-02212-3. Cancer Cell Int. 2021. PMID: 34556138 Free PMC article.
-
Role of LGMN in tumor development and its progression and connection with the tumor microenvironment.Front Mol Biosci. 2023 Feb 7;10:1121964. doi: 10.3389/fmolb.2023.1121964. eCollection 2023. Front Mol Biosci. 2023. PMID: 36825203 Free PMC article. Review.
-
TMEM60 Promotes the Proliferation and Migration and Inhibits the Apoptosis of Glioma through Modulating AKT Signaling.J Oncol. 2022 Jan 3;2022:9913700. doi: 10.1155/2022/9913700. eCollection 2022. J Oncol. 2022. Retraction in: J Oncol. 2023 Jan 23;2023:9767535. doi: 10.1155/2023/9767535. PMID: 35027926 Free PMC article. Retracted.
-
The Asparaginyl Endopeptidase Legumain: An Emerging Therapeutic Target and Potential Biomarker for Alzheimer's Disease.Int J Mol Sci. 2022 Sep 6;23(18):10223. doi: 10.3390/ijms231810223. Int J Mol Sci. 2022. PMID: 36142134 Free PMC article. Review.
-
The Mammalian Cysteine Protease Legumain in Health and Disease.Int J Mol Sci. 2022 Dec 15;23(24):15983. doi: 10.3390/ijms232415983. Int J Mol Sci. 2022. PMID: 36555634 Free PMC article. Review.
References
-
- Comel A, Sorrentino G, Capaci V, et al. The cytoplasmic side of p53’s oncosuppressive activities. FEBS Lett. 2014;588(16):2600–2609. - PubMed
-
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

