The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis
- PMID: 31398940
- PMCID: PMC6719901
- DOI: 10.3390/ijms20163876
The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis
Abstract
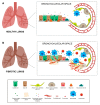
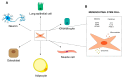
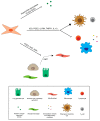
Radiation therapy is one of the most important treatment modalities for thoracic tumors. Despite significant advances in radiation techniques, radiation-induced lung injury (RILI) still occurs in up to 30% of patients undergoing thoracic radiotherapy, and therefore remains the main dose-limiting obstacle. RILI is a potentially lethal clinical complication of radiotherapy that has 2 main stages: an acute stage defined as radiation pneumonitis, and a late stage defined as radiation-induced lung fibrosis. Patients who develop lung fibrosis have a reduced quality of life with progressive and irreversible organ malfunction. Currently, the most effective intervention for the treatment of lung fibrosis is lung transplantation, but the lack of available lungs and transplantation-related complications severely limits the success of this procedure. Over the last few decades, advances have been reported in the use of mesenchymal stem cells (MSCs) for lung tissue repair and regeneration. MSCs not only replace damaged lung epithelial cells but also promote tissue repair through the secretion of anti-inflammatory and anti-fibrotic factors. Here, we present an overview of MSC-based therapy for radiation-induced lung fibrosis, focusing in particular on the molecular mechanisms involved and describing the most recent preclinical and clinical studies carried out in the field.
Keywords: lung fibrosis; mesenchymal stem cells (MSCs); radiotherapy; regenerative medicine; thoracic cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Applications and therapeutic mechanisms of action of mesenchymal stem cells in radiation-induced lung injury.Stem Cell Res Ther. 2021 Mar 25;12(1):212. doi: 10.1186/s13287-021-02279-9. Stem Cell Res Ther. 2021. PMID: 33766127 Free PMC article. Review.
-
Mesenchymal stem cell-based therapy for radiation-induced lung injury.Stem Cell Res Ther. 2018 Jan 31;9(1):18. doi: 10.1186/s13287-018-0776-6. Stem Cell Res Ther. 2018. PMID: 29386045 Free PMC article. Review.
-
Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis.Stem Cell Res Ther. 2015 May 20;6(1):97. doi: 10.1186/s13287-015-0081-6. Stem Cell Res Ther. 2015. PMID: 25986930 Free PMC article.
-
Optimization of the adipose-derived mesenchymal stem cell delivery time for radiation-induced lung fibrosis treatment in rats.Sci Rep. 2019 Apr 3;9(1):5589. doi: 10.1038/s41598-019-41576-5. Sci Rep. 2019. PMID: 30944348 Free PMC article.
-
Extracellular superoxide dismutase increased the therapeutic potential of human mesenchymal stromal cells in radiation pulmonary fibrosis.Cytotherapy. 2017 May;19(5):586-602. doi: 10.1016/j.jcyt.2017.02.359. Epub 2017 Mar 15. Cytotherapy. 2017. PMID: 28314668
Cited by
-
The Role of Pulmonary Surfactant Phospholipids in Fibrotic Lung Diseases.Int J Mol Sci. 2022 Dec 25;24(1):326. doi: 10.3390/ijms24010326. Int J Mol Sci. 2022. PMID: 36613771 Free PMC article. Review.
-
Advances of mesenchymal stem cells and their derived extracellular vesicles as a promising therapy for acute respiratory distress syndrome: from bench to clinic.Front Immunol. 2023 Aug 29;14:1244930. doi: 10.3389/fimmu.2023.1244930. eCollection 2023. Front Immunol. 2023. PMID: 37711624 Free PMC article. Review.
-
Targets for protection and mitigation of radiation injury.Cell Mol Life Sci. 2020 Aug;77(16):3129-3159. doi: 10.1007/s00018-020-03479-x. Epub 2020 Feb 18. Cell Mol Life Sci. 2020. PMID: 32072238 Free PMC article. Review.
-
Applications and therapeutic mechanisms of action of mesenchymal stem cells in radiation-induced lung injury.Stem Cell Res Ther. 2021 Mar 25;12(1):212. doi: 10.1186/s13287-021-02279-9. Stem Cell Res Ther. 2021. PMID: 33766127 Free PMC article. Review.
-
Emerging Roles of Aldehyde Dehydrogenase Isoforms in Anti-cancer Therapy Resistance.Front Med (Lausanne). 2022 Mar 1;9:795762. doi: 10.3389/fmed.2022.795762. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35299840 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

