Sex Differences in Adrenal Bmal1 Deletion-Induced Augmentation of Glucocorticoid Responses to Stress and ACTH in Mice
- PMID: 31398249
- PMCID: PMC6735739
- DOI: 10.1210/en.2019-00357
Sex Differences in Adrenal Bmal1 Deletion-Induced Augmentation of Glucocorticoid Responses to Stress and ACTH in Mice
Abstract
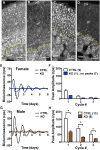
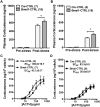
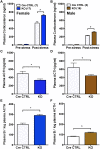
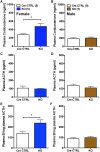
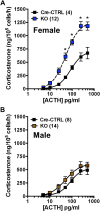
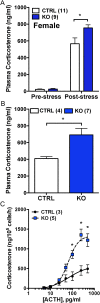
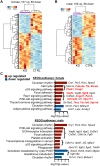
The circadian glucocorticoid (GC) rhythm is dependent on a molecular clock in the suprachiasmatic nucleus (SCN) and an adrenal clock that is synchronized by the SCN. To determine whether the adrenal clock modulates GC responses to stress, experiments used female and male Cyp11A1Cre/+::Bmal1Fl/Fl knockout [side-chain cleavage (SCC)-KO] mice, in which the core clock gene, Bmal1, is deleted in all steroidogenic tissues, including the adrenal cortex. Following restraint stress, female and male SCC-KO mice demonstrate augmented plasma corticosterone but not plasma ACTH. In contrast, following submaximal scruff stress, plasma corticosterone was elevated only in female SCC-KO mice. Adrenal sensitivity to ACTH was measured in vitro using acutely dispersed adrenocortical cells. Maximal corticosterone responses to ACTH were elevated in cells from female KO mice without affecting the EC50 response. Neither the maximum nor the EC50 response to ACTH was affected in male cells, indicating that female SCC-KO mice show a stronger adrenal phenotype. Parallel experiments were conducted using female Cyp11B2 (Aldosterone Synthase)Cre/+::Bmal1Fl/Fl mice and adrenal cortex-specific Bmal1-null (Ad-KO) mice. Plasma corticosterone was increased in Ad-KO mice following restraint or scruff stress, and in vitro responses to ACTH were elevated in adrenal cells from Ad-KO mice, replicating data from female SCC-KO mice. Gene analysis showed increased expression of adrenal genes in female SCC-KO mice involved in cell cycle control, cell adhesion-extracellular matrix interaction, and ligand receptor activity that could promote steroid production. These observations underscore a role for adrenal Bmal1 as an attenuator of steroid secretion that is most prominent in female mice.
Copyright © 2019 Endocrine Society.
Conflict of interest statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Figures

Similar articles
-
The Adrenal Clock Prevents Aberrant Light-Induced Alterations in Circadian Glucocorticoid Rhythms.Endocrinology. 2018 Dec 1;159(12):3950-3964. doi: 10.1210/en.2018-00769. Endocrinology. 2018. PMID: 30321360 Free PMC article.
-
Dissociation of Molecular and Endocrine Circadian Rhythms in Male Mice Lacking Bmal1 in the Adrenal Cortex.Endocrinology. 2016 Nov;157(11):4222-4233. doi: 10.1210/en.2016-1330. Epub 2016 Oct 3. Endocrinology. 2016. PMID: 27690690
-
Altered rhythm of adrenal clock genes, StAR and serum corticosterone in VIP receptor 2-deficient mice.J Mol Neurosci. 2012 Nov;48(3):584-96. doi: 10.1007/s12031-012-9804-7. Epub 2012 May 24. J Mol Neurosci. 2012. PMID: 22622901
-
[The roles of clock genes in obesity].Nihon Rinsho. 2013 Feb;71(2):244-8. Nihon Rinsho. 2013. PMID: 23631200 Review. Japanese.
-
ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex.Microsc Res Tech. 2003 Jun 15;61(3):300-7. doi: 10.1002/jemt.10339. Microsc Res Tech. 2003. PMID: 12768545 Review.
Cited by
-
Does a Red House Affect Rhythms in Mice with a Corrupted Circadian System?Int J Mol Sci. 2021 Feb 25;22(5):2288. doi: 10.3390/ijms22052288. Int J Mol Sci. 2021. PMID: 33669004 Free PMC article.
-
Interactions Between Gut Microbiota and Acute Restraint Stress in Peripheral Structures of the Hypothalamic-Pituitary-Adrenal Axis and the Intestine of Male Mice.Front Immunol. 2019 Nov 19;10:2655. doi: 10.3389/fimmu.2019.02655. eCollection 2019. Front Immunol. 2019. PMID: 31798585 Free PMC article.
-
Circadian rhythm disruption and endocrine-related tumors.World J Clin Oncol. 2024 Jul 24;15(7):818-834. doi: 10.5306/wjco.v15.i7.818. World J Clin Oncol. 2024. PMID: 39071458 Free PMC article. Review.
-
Adrenal cortex renewal in health and disease.Nat Rev Endocrinol. 2021 Jul;17(7):421-434. doi: 10.1038/s41574-021-00491-4. Epub 2021 May 19. Nat Rev Endocrinol. 2021. PMID: 34011989 Review.
-
Cross-species physiological interactions of endocrine disrupting chemicals with the circadian clock.Gen Comp Endocrinol. 2021 Jan 15;301:113650. doi: 10.1016/j.ygcen.2020.113650. Epub 2020 Nov 7. Gen Comp Endocrinol. 2021. PMID: 33166531 Free PMC article. Review.
References
-
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4(2):163–173. - PubMed
-
- Dumbell R, Leliavski A, Matveeva O, Blaum C, Tsang AH, Oster H. Dissociation of molecular and endocrine circadian rhythms in male mice lacking Bmal1 in the adrenal cortex. Endocrinology. 2016;157(11):4222–4233. - PubMed
-
- Kloehn I, Pillai SB, Officer L, Klement C, Gasser PJ, Evans JA. Sexual differentiation of circadian clock function in the adrenal gland. Endocrinology. 2016;157(5):1895–1904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

