Meiotic cellular rejuvenation is coupled to nuclear remodeling in budding yeast
- PMID: 31397671
- PMCID: PMC6711709
- DOI: 10.7554/eLife.47156
Meiotic cellular rejuvenation is coupled to nuclear remodeling in budding yeast
Abstract
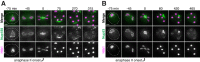
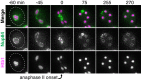
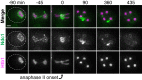
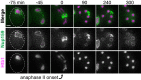
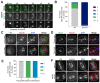
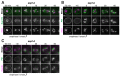
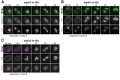
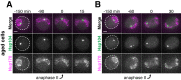
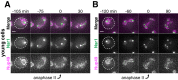
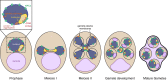
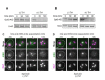
Production of healthy gametes in meiosis relies on the quality control and proper distribution of both nuclear and cytoplasmic contents. Meiotic differentiation naturally eliminates age-induced cellular damage by an unknown mechanism. Using time-lapse fluorescence microscopy in budding yeast, we found that nuclear senescence factors - including protein aggregates, extrachromosomal ribosomal DNA circles, and abnormal nucleolar material - are sequestered away from chromosomes during meiosis II and subsequently eliminated. A similar sequestration and elimination process occurs for the core subunits of the nuclear pore complex in both young and aged cells. Nuclear envelope remodeling drives the formation of a membranous compartment containing the sequestered material. Importantly, de novo generation of plasma membrane is required for the sequestration event, preventing the inheritance of long-lived nucleoporins and senescence factors into the newly formed gametes. Our study uncovers a new mechanism of nuclear quality control and provides insight into its function in meiotic cellular rejuvenation.
Keywords: S. cerevisiae; aging; cell biology; meiosis; nuclear pore complex; nucleolus; protein aggregation; quality control.
© 2019, King et al.
Conflict of interest statement
GK, JG, JS, KC, DJ, KM No competing interests declared, EÜ Reviewing editor, eLife
Figures




Similar articles
-
The dynamic nuclear periphery as a facilitator of gamete health and rejuvenation.Curr Genet. 2020 Jun;66(3):487-493. doi: 10.1007/s00294-019-01050-1. Epub 2020 Jan 8. Curr Genet. 2020. PMID: 31915924 Free PMC article. Review.
-
Forever young: the key to rejuvenation during gametogenesis.Curr Genet. 2021 Apr;67(2):231-235. doi: 10.1007/s00294-020-01133-4. Epub 2020 Nov 27. Curr Genet. 2021. PMID: 33247310 Free PMC article. Review.
-
The ESCRT-III complex is required for nuclear pore complex sequestration and regulates gamete replicative lifespan in budding yeast meiosis.Nucleus. 2020 Dec;11(1):219-236. doi: 10.1080/19491034.2020.1812872. Nucleus. 2020. PMID: 32893723 Free PMC article.
-
Effects of age on meiosis in budding yeast.Dev Cell. 2009 Jun;16(6):844-55. doi: 10.1016/j.devcel.2009.05.013. Dev Cell. 2009. PMID: 19531355 Free PMC article.
-
Interorganelle interactions and inheritance patterns of nuclei and vacuoles in budding yeast meiosis.Autophagy. 2014 Feb;10(2):285-95. doi: 10.4161/auto.27192. Epub 2013 Dec 4. Autophagy. 2014. PMID: 24345927 Free PMC article.
Cited by
-
Molding immortality from a plastic germline.Curr Opin Cell Biol. 2021 Dec;73:1-8. doi: 10.1016/j.ceb.2021.04.010. Epub 2021 Jun 3. Curr Opin Cell Biol. 2021. PMID: 34091218 Free PMC article. Review.
-
The middle domain of Hsp104 can ensure substrates are functional after processing.PLoS Genet. 2024 Oct 3;20(10):e1011424. doi: 10.1371/journal.pgen.1011424. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39361717 Free PMC article.
-
Regulation of organelle size and organization during development.Semin Cell Dev Biol. 2023 Jan 15;133:53-64. doi: 10.1016/j.semcdb.2022.02.002. Epub 2022 Feb 8. Semin Cell Dev Biol. 2023. PMID: 35148938 Free PMC article. Review.
-
A distinct inner nuclear membrane proteome in Saccharomyces cerevisiae gametes.G3 (Bethesda). 2021 Dec 8;11(12):jkab345. doi: 10.1093/g3journal/jkab345. G3 (Bethesda). 2021. PMID: 34849801 Free PMC article.
-
The transcriptional regulator Ume6 is a major driver of early gene expression during gametogenesis.Genetics. 2023 Oct 4;225(2):iyad123. doi: 10.1093/genetics/iyad123. Genetics. 2023. PMID: 37431893 Free PMC article.
References
-
- Aebi M, Clark MW, Vijayraghavan U, Abelson J. A yeast mutant, PRP20, altered in mRNA metabolism and maintenance of the nuclear structure, is defective in a gene homologous to the human gene RCC1 which is involved in the control of chromosome condensation. MGG Molecular & General Genetics. 1990;224:72–80. doi: 10.1007/BF00259453. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

