Evaluation of the Antimicrobial Peptide, RP557, for the Broad-Spectrum Treatment of Wound Pathogens and Biofilm
- PMID: 31396193
- PMCID: PMC6667648
- DOI: 10.3389/fmicb.2019.01688
Evaluation of the Antimicrobial Peptide, RP557, for the Broad-Spectrum Treatment of Wound Pathogens and Biofilm
Abstract
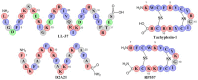
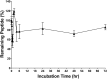
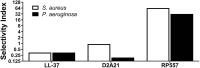
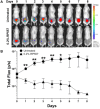
The relentless growth of multidrug resistance and generation of recalcitrant biofilm are major obstacles in treating wounds, particularly in austere military environments where broad-spectrum pathogen coverage is needed. Designed antimicrobial peptides (dAMPs) are constructed analogs of naturally occurring AMPs that provide the first line of defense in many organisms. RP557 is a dAMP resulting from iterative rational chemical structural analoging with endogenous AMPs, human cathelicidin LL-37 and Tachyplesin 1 and the synthetic D2A21 used as structural benchmarks. RP557 possesses broad spectrum activity against Gram-positive and Gram-negative bacteria and fungi, including recalcitrant biofilm with substantial selective killing over bacterial cells compared to mammalian cells. RP557 did not induce resistance following chronic passages of Pseudomonas aeruginosa and Staphylococcus aureus at subinhibitory concentrations, whereas concurrently run conventional antibiotics, gentamycin, and clindamycin, did. Furthermore, RP557 was able to subsequently eliminate the generated gentamycin resistant P. aeruginosa and clindamycin resistant S. aureus strains without requiring an increase in minimum inhibitory concentration (MIC) concentrations. RP557 was evaluated further in a MRSA murine wound abrasion infection model with a topical application of 0.2% RP557, completely eliminating infection. If these preclinical results are translated into the clinical setting, RP557 may become crucial for the empirical broad-spectrum treatment of wound pathogens, so that infections can be reduced to a preventable complication of combat-related injuries.
Keywords: antibacterial; antimicrobial peptides; bacterial resistance; biofilm; multidrug resistance; wound infection.
Figures


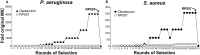

Similar articles
-
Effects of synthetic peptide RP557 and its origin, LL-37, on carbapenem-resistant Pseudomonas aeruginosa.Microbiol Spectr. 2023 Aug 9;11(5):e0043023. doi: 10.1128/spectrum.00430-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37555659 Free PMC article.
-
A Designed Host Defense Peptide for the Topical Treatment of MRSA-Infected Diabetic Wounds.Int J Mol Sci. 2023 Jan 21;24(3):2143. doi: 10.3390/ijms24032143. Int J Mol Sci. 2023. PMID: 36768463 Free PMC article.
-
Designed Antimicrobial Peptides for Topical Treatment of Antibiotic Resistant Acne Vulgaris.Antibiotics (Basel). 2020 Jan 13;9(1):23. doi: 10.3390/antibiotics9010023. Antibiotics (Basel). 2020. PMID: 31940992 Free PMC article.
-
Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds.Front Pharmacol. 2018 Mar 28;9:281. doi: 10.3389/fphar.2018.00281. eCollection 2018. Front Pharmacol. 2018. PMID: 29643807 Free PMC article. Review.
-
Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria.J Microbiol Immunol Infect. 2017 Aug;50(4):405-410. doi: 10.1016/j.jmii.2016.12.005. Epub 2017 Jun 26. J Microbiol Immunol Infect. 2017. PMID: 28690026 Review.
Cited by
-
A truncated peptide Spgillcin177-189 derived from mud crab Scylla paramamosain exerting multiple antibacterial activities.Front Cell Infect Microbiol. 2022 Aug 2;12:928220. doi: 10.3389/fcimb.2022.928220. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36061863 Free PMC article.
-
Designed Antimicrobial Peptides Against Trauma-Related Cutaneous Invasive Fungal Wound Infections.J Fungi (Basel). 2020 Sep 22;6(3):184. doi: 10.3390/jof6030184. J Fungi (Basel). 2020. PMID: 32971819 Free PMC article.
-
Active compounds in kepok banana peel as anti-inflammatory in acne vulgaris: Review article.Ann Med Surg (Lond). 2022 Nov 12;84:104868. doi: 10.1016/j.amsu.2022.104868. eCollection 2022 Dec. Ann Med Surg (Lond). 2022. PMID: 36582904 Free PMC article. Review.
-
The Protective Effects of Cath-MH With Anti-Propionibacterium Acnes and Anti-Inflammation Functions on Acne Vulgaris.Front Pharmacol. 2021 Dec 9;12:788358. doi: 10.3389/fphar.2021.788358. eCollection 2021. Front Pharmacol. 2021. PMID: 34955858 Free PMC article.
-
Antimicrobial Proteins: Structure, Molecular Action, and Therapeutic Potential.Pharmaceutics. 2022 Dec 26;15(1):72. doi: 10.3390/pharmaceutics15010072. Pharmaceutics. 2022. PMID: 36678702 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

