CaM Kinase: Still Inspiring at 40
- PMID: 31394063
- PMCID: PMC6688632
- DOI: 10.1016/j.neuron.2019.05.033
CaM Kinase: Still Inspiring at 40
Abstract
The Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) was touted as a memory molecule, even before its involvement in long-term potentiation (LTP) was shown. The enzyme has not disappointed, with subsequent demonstrations of remarkable structural and regulatory properties. Its neuronal functions now extend to long-term depression (LTD), and last year saw the first direct evidence for memory storage by CaMKII. Although CaMKII may have taken the spotlight, it is a member of a large family of diverse and interesting CaM kinases. Our aim is to place CaMKII in context of the other CaM kinases and then review certain aspects of this kinase that are of current interest.
Keywords: CaMKII; DAPK; LTD; LTP; calmodulin; memory; synapse.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
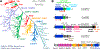






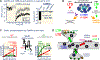
Similar articles
-
CaMKII regulation in information processing and storage.Trends Neurosci. 2012 Oct;35(10):607-18. doi: 10.1016/j.tins.2012.05.003. Epub 2012 Jun 19. Trends Neurosci. 2012. PMID: 22717267 Free PMC article. Review.
-
DAPK1 Mediates LTD by Making CaMKII/GluN2B Binding LTP Specific.Cell Rep. 2017 Jun 13;19(11):2231-2243. doi: 10.1016/j.celrep.2017.05.068. Cell Rep. 2017. PMID: 28614711 Free PMC article.
-
Modelling the dynamics of CaMKII-NMDAR complex related to memory formation in synapses: the possible roles of threonine 286 autophosphorylation of CaMKII in long term potentiation.J Theor Biol. 2015 Jan 21;365:403-19. doi: 10.1016/j.jtbi.2014.11.001. Epub 2014 Nov 11. J Theor Biol. 2015. PMID: 25446714
-
CaMKII regulates the depalmitoylation and synaptic removal of the scaffold protein AKAP79/150 to mediate structural long-term depression.J Biol Chem. 2018 Feb 2;293(5):1551-1567. doi: 10.1074/jbc.M117.813808. Epub 2017 Dec 1. J Biol Chem. 2018. PMID: 29196604 Free PMC article. Review.
-
Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation.J Neurosci. 2005 Feb 2;25(5):1281-90. doi: 10.1523/JNEUROSCI.4086-04.2005. J Neurosci. 2005. PMID: 15689566 Free PMC article.
Cited by
-
CaMKII: a central molecular organizer of synaptic plasticity, learning and memory.Nat Rev Neurosci. 2022 Nov;23(11):666-682. doi: 10.1038/s41583-022-00624-2. Epub 2022 Sep 2. Nat Rev Neurosci. 2022. PMID: 36056211 Review.
-
Untranslated regions of brain-derived neurotrophic factor mRNA control its translatability and subcellular localization.J Biol Chem. 2023 Feb;299(2):102897. doi: 10.1016/j.jbc.2023.102897. Epub 2023 Jan 11. J Biol Chem. 2023. PMID: 36639028 Free PMC article.
-
LTP induction by structural rather than enzymatic functions of CaMKII.Nature. 2023 Sep;621(7977):146-153. doi: 10.1038/s41586-023-06465-y. Epub 2023 Aug 30. Nature. 2023. PMID: 37648853 Free PMC article.
-
Downregulation of mGluR1-mediated signaling underlying autistic-like core symptoms in Shank1 P1812L-knock-in mice.Transl Psychiatry. 2023 Oct 25;13(1):329. doi: 10.1038/s41398-023-02626-9. Transl Psychiatry. 2023. PMID: 37880287 Free PMC article.
-
Multivalent electrostatic pi-cation interaction between synaptophysin and synapsin is responsible for the coacervation.Mol Brain. 2021 Sep 8;14(1):137. doi: 10.1186/s13041-021-00846-y. Mol Brain. 2021. PMID: 34496937 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

