Eluted 25-hydroxyvitamin D3 from radially aligned nanofiber scaffolds enhances cathelicidin production while reducing inflammatory response in human immune system-engrafted mice
- PMID: 31386930
- PMCID: PMC6801031
- DOI: 10.1016/j.actbio.2019.08.005
Eluted 25-hydroxyvitamin D3 from radially aligned nanofiber scaffolds enhances cathelicidin production while reducing inflammatory response in human immune system-engrafted mice
Abstract
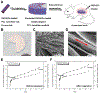
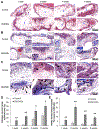
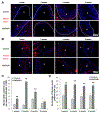
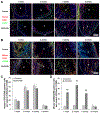
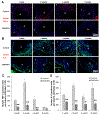
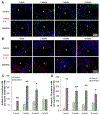
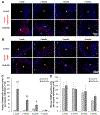
Vitamin D3 modulates immune response, induces endogenous antimicrobial peptide production, and enhances innate immunity to defend against infections. These findings suggest that incorporating vitamin D3 into medical devices or scaffolds could positively modulate host immune response and prevent infections. In the current study, we evaluated host responses and endogenous antimicrobial peptide production using 25-hydroxyvitamin D3 (25(OH)D3)-eluting radially aligned PCL nanofiber scaffolds in human immune system-engrafted mice. We transformed traditional 2D electrospun nanofiber membranes into radially aligned PCL nanofiber scaffolds using the concept of solid of revolution and an innovative gas-foaming technique. Such scaffolds can promote rapid cellular infiltration and neovascularization. The infiltrating immune cells within subcutaneously implanted 25(OH)D3-containing scaffolds mainly consisted of human macrophages in the M1 phase (CCR7+), mice macrophages in the M2 phase (CD206+), and human cytotoxic T cells (CD8+) other than few human T-helper cells (CD4+). The 25(OH)D3-eluting nanofiber scaffolds significantly inhibited the production of pro-inflammatory cytokines (TNF-α, IL-6), while accelerating the production of anti-inflammatory cytokines (IL-4, IL-10) within the scaffolds. Additionally, we observed increased expression of human cathelicidin LL-37 within the 25(OH)D3-eluting scaffolds, while no LL-37 expression was observed in the control. Together, these findings support further work in the design of vitamin D3-eluting medical devices or scaffolds for modulating immune response and promoting antimicrobial peptide production. This could potentially reduce the inflammatory response, prevent infections, and eventually improve success rates of implants. STATEMENT OF SIGNIFICANCE: Transplant failure of medical devices, grafts, scaffolds, and tissue-engineered constructs due to inflammation and infection causes not only economic losses but also sufferings of second operation to the patient. Positive modulation of the host response to implants, scaffolds, and tissue-engineered constructs is likely to reduce the failure rate. Vitamin D3 plays an important role in modulating the immune response. It is able to not only reduce inflammation and induce endogenous antimicrobial peptide production but also prevent multidrug resistance and other side effects of traditional antibiotics. In this study, host responses to 25-hydroxyvitamin D3 (25(OH)D3)-eluting radially aligned PCL nanofiber scaffolds were evaluated in human immune system-engrafted mice. The 25(OH)D3-eluting medical devices or scaffolds were able to modulate positive immune response and promote antimicrobial peptide production. This work presented an innate immunity-enhancing approach for reducing the inflammatory response and preventing infections, likely resulting in improvement of success rates of implants.
Keywords: 25-Hydroxyvitamin D(3); Cathelicidin production; Inflammatory response; Radially aligned nanofiber scaffolds; Sustained release.
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare no conflict of interest.
Figures
Similar articles
-
Nanofiber-based sutures induce endogenous antimicrobial peptide.Nanomedicine (Lond). 2017 Nov;12(21):2597-2609. doi: 10.2217/nnm-2017-0161. Epub 2017 Sep 29. Nanomedicine (Lond). 2017. PMID: 28960168 Free PMC article.
-
Codelivery of 1α,25-Dihydroxyvitamin D3 and CYP24A1 Inhibitor VID400 by Nanofiber Dressings Promotes Endogenous Antimicrobial Peptide LL-37 Induction.Mol Pharm. 2022 Mar 7;19(3):974-984. doi: 10.1021/acs.molpharmaceut.1c00944. Epub 2022 Feb 18. Mol Pharm. 2022. PMID: 35179903 Free PMC article.
-
CO2-expanded nanofiber scaffolds maintain activity of encapsulated bioactive materials and promote cellular infiltration and positive host response.Acta Biomater. 2018 Mar 1;68:237-248. doi: 10.1016/j.actbio.2017.12.018. Epub 2017 Dec 19. Acta Biomater. 2018. PMID: 29269334 Free PMC article.
-
Vitamin D3 modulates the innate immune response through regulation of the hCAP-18/LL-37 gene expression and cytokine production.Inflamm Res. 2016 Jan;65(1):25-32. doi: 10.1007/s00011-015-0884-z. Epub 2015 Oct 3. Inflamm Res. 2016. PMID: 26433491 Review.
-
The Active Metabolite of Vitamin D3 as a Potential Immunomodulator.Scand J Immunol. 2016 Feb;83(2):83-91. doi: 10.1111/sji.12403. Scand J Immunol. 2016. PMID: 26678915 Review.
Cited by
-
Electrospun Nanofibers for Wound Management.ChemNanoMat. 2022 Jul;8(7):e202100349. doi: 10.1002/cnma.202100349. Epub 2021 Nov 1. ChemNanoMat. 2022. PMID: 35990019 Free PMC article.
-
Naringenin is a Potential Anabolic Treatment for Bone Loss by Modulating Osteogenesis, Osteoclastogenesis, and Macrophage Polarization.Front Pharmacol. 2022 May 2;13:872188. doi: 10.3389/fphar.2022.872188. eCollection 2022. Front Pharmacol. 2022. PMID: 35586056 Free PMC article.
-
Identification of a humanized mouse model for functional testing of immune-mediated biomaterial foreign body response.Sci Adv. 2023 Jun 16;9(24):eade9488. doi: 10.1126/sciadv.ade9488. Epub 2023 Jun 16. Sci Adv. 2023. PMID: 37327334 Free PMC article.
-
Scaffold-Mediated Immunoengineering as Innovative Strategy for Tendon Regeneration.Cells. 2022 Jan 13;11(2):266. doi: 10.3390/cells11020266. Cells. 2022. PMID: 35053383 Free PMC article. Review.
-
Hierarchically Assembled Nanofiber Scaffolds with Dual Growth Factor Gradients Promote Skin Wound Healing Through Rapid Cell Recruitment.Adv Sci (Weinh). 2024 Apr;11(14):e2309993. doi: 10.1002/advs.202309993. Epub 2024 Feb 7. Adv Sci (Weinh). 2024. PMID: 38326085 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

