HIV-1 Protease Inhibitors Incorporating Stereochemically Defined P2' Ligands To Optimize Hydrogen Bonding in the Substrate Envelope
- PMID: 31386368
- PMCID: PMC6941148
- DOI: 10.1021/acs.jmedchem.9b00838
HIV-1 Protease Inhibitors Incorporating Stereochemically Defined P2' Ligands To Optimize Hydrogen Bonding in the Substrate Envelope
Abstract
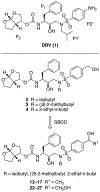
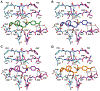
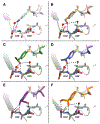
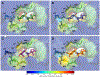
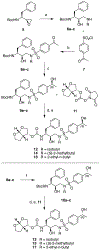
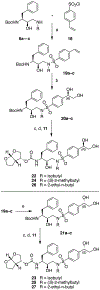
A structure-guided design strategy was used to improve the resistance profile of HIV-1 protease inhibitors by optimizing hydrogen bonding and van der Waals interactions with the protease while staying within the substrate envelope. Stereoisomers of 4-(1-hydroxyethyl)benzene and 4-(1,2-dihydroxyethyl)benzene moieties were explored as P2' ligands providing pairs of diastereoisomers epimeric at P2', which exhibited distinct potency profiles depending on the configuration of the hydroxyl group and size of the P1' group. While compounds with the 4-(1-hydroxyethyl)benzene P2' moiety maintained excellent antiviral potency against a panel of multidrug-resistant HIV-1 strains, analogues with the polar 4-(1,2-dihydroxyethyl)benzene moiety were less potent, and only the (R)-epimer incorporating a larger 2-ethylbutyl P1' group showed improved potency. Crystal structures of protease-inhibitor complexes revealed strong hydrogen bonding interactions of both (R)- and (S)-stereoisomers of the hydroxyethyl group with Asp30'. Notably, the (R)-dihydroxyethyl group was involved in a unique pattern of direct hydrogen bonding interactions with the backbone amides of Asp29' and Asp30'. The SAR data and analysis of crystal structures provide insights for optimizing these promising HIV-1 protease inhibitors.
Figures
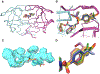
Similar articles
-
Novel HIV-1 protease inhibitors (PIs) containing a bicyclic P2 functional moiety, tetrahydropyrano-tetrahydrofuran, that are potent against multi-PI-resistant HIV-1 variants.Antimicrob Agents Chemother. 2011 Apr;55(4):1717-27. doi: 10.1128/AAC.01540-10. Epub 2011 Jan 31. Antimicrob Agents Chemother. 2011. PMID: 21282450 Free PMC article.
-
Triterpene esters from Uncaria rhynchophylla hooks as potent HIV-1 protease inhibitors and their molecular docking study.Sci Rep. 2024 Dec 30;14(1):31576. doi: 10.1038/s41598-024-76551-2. Sci Rep. 2024. PMID: 39738211 Free PMC article.
-
Molecular Determinants of Epistasis in HIV-1 Protease: Elucidating the Interdependence of L89V and L90M Mutations in Resistance.Biochemistry. 2019 Sep 3;58(35):3711-3726. doi: 10.1021/acs.biochem.9b00446. Epub 2019 Aug 19. Biochemistry. 2019. PMID: 31386353 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Male circumcision for prevention of heterosexual acquisition of HIV in men.Cochrane Database Syst Rev. 2009 Apr 15;2009(2):CD003362. doi: 10.1002/14651858.CD003362.pub2. Cochrane Database Syst Rev. 2009. PMID: 19370585 Free PMC article. Review.
Cited by
-
Viral proteases: Structure, mechanism and inhibition.Enzymes. 2021;50:301-333. doi: 10.1016/bs.enz.2021.09.004. Epub 2021 Nov 17. Enzymes. 2021. PMID: 34861941 Free PMC article.
-
Piperidine scaffold as the novel P2-ligands in cyclopropyl-containing HIV-1 protease inhibitors: Structure-based design, synthesis, biological evaluation and docking study.PLoS One. 2020 Jul 22;15(7):e0235483. doi: 10.1371/journal.pone.0235483. eCollection 2020. PLoS One. 2020. PMID: 32697773 Free PMC article.
-
Structural Analysis of Potent Hybrid HIV-1 Protease Inhibitors Containing Bis-tetrahydrofuran in a Pseudosymmetric Dipeptide Isostere.J Med Chem. 2020 Aug 13;63(15):8296-8313. doi: 10.1021/acs.jmedchem.0c00529. Epub 2020 Aug 3. J Med Chem. 2020. PMID: 32672965 Free PMC article.
-
HIV-1 protease inhibitors with a P1 phosphonate modification maintain potency against drug-resistant variants by increased interactions with flap residues.Eur J Med Chem. 2023 Sep 5;257:115501. doi: 10.1016/j.ejmech.2023.115501. Epub 2023 May 18. Eur J Med Chem. 2023. PMID: 37244161 Free PMC article.
-
Elucidation of the α-Ketoamide Inhibition Mechanism: Revealing the Critical Role of the Electrostatic Reorganization Effect of Asp17 in the Active Site of the 20S Proteasome.ACS Catal. 2023 Nov 3;13(21):14368-14376. doi: 10.1021/acscatal.3c03538. Epub 2023 Oct 25. ACS Catal. 2023. PMID: 39188993 Free PMC article.
References
-
- Cihlar T; Fordyce M Current status and prospects of HIV treatment. Curr. Opin. Virol 2016, 18, 50–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Miscellaneous

