Update to the study protocol, including statistical analysis plan, for the multicentre, randomised controlled OuTSMART trial: a combined screening/treatment programme to prevent premature failure of renal transplants due to chronic rejection in patients with HLA antibodies
- PMID: 31383029
- PMCID: PMC6683506
- DOI: 10.1186/s13063-019-3602-2
Update to the study protocol, including statistical analysis plan, for the multicentre, randomised controlled OuTSMART trial: a combined screening/treatment programme to prevent premature failure of renal transplants due to chronic rejection in patients with HLA antibodies
Abstract
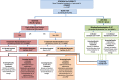
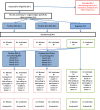
Background: Chronic rejection is the single biggest cause of premature kidney graft failure. HLA antibodies (Ab) are an established prognostic biomarker for premature graft failure so there is a need to test whether treatment decisions based on the presence of the biomarker can alter prognosis. The Optimised TacrolimuS and MMF for HLA Antibodies after Renal Transplantation (OuTSMART) trial combines two elements. Firstly, testing whether a routine screening programme for HLA Ab in all kidney transplant recipients is useful by comparing blinding versus unblinding of HLA Ab status. Secondly, for those found to be HLA Ab+, testing whether the introduction of a standard optimisation treatment protocol can reduce graft failure rates.
Methods: OuTSMART is a prospective, open-labelled, randomised biomarker-based strategy (hybrid) trial, with two arms stratified by biomarker (HLA Ab) status. The primary outcome was amended from graft failure rates at 3 years to time to graft failure to increase power and require fewer participants to be recruited. Length of follow-up subsequently is variable, with all participants followed up for at least 43 months up to a maximum of 89 months. The primary outcome will be analysed using Cox regression adjusting for stratification factors. Analyses will be according to the intention-to-treat using all participants as randomised. Outcomes will be analysed comparing standard care versus biomarker-led care groups within the HLA Ab+ participants (including those who become HLA Ab+ through re-screening) as well as between HLA-Ab-unblinded and HLA-Ab-blinded groups using all participants.
Discussion: Changes to the primary outcome permit recruitment of fewer participants to achieve the same statistical power. Pre-stating the statistical analysis plan guards against changes to the analysis methods at the point of analysis that might otherwise introduce bias through knowledge of the data. Any deviations from the analysis plan will be justified in the final report.
Trial registration: ISRCTN registry, ID: ISRCTN46157828 . Registered on 26 March 2013; EudraCT 2012-004308-36 . Registered on 10 December 2012.
Keywords: Graft failure; Human leucocyte antigen antibodies; Immunosuppression; Randomised controlled trial; Renal transplantation; Statistical analysis plan; Time-to-event.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Optimized immunosuppression to prevent graft failure in renal transplant recipients with HLA antibodies (OuTSMART): a randomised controlled trial.EClinicalMedicine. 2023 Jan 12;56:101819. doi: 10.1016/j.eclinm.2022.101819. eCollection 2023 Feb. EClinicalMedicine. 2023. PMID: 36684392 Free PMC article.
-
Can a combined screening/treatment programme prevent premature failure of renal transplants due to chronic rejection in patients with HLA antibodies: study protocol for the multicentre randomised controlled OuTSMART trial.Trials. 2014 Jan 21;15:30. doi: 10.1186/1745-6215-15-30. Trials. 2014. PMID: 24447519 Free PMC article. Clinical Trial.
-
Preventing kidney transplant failure by screening for antibodies against human leucocyte antigens followed by optimised immunosuppression: OuTSMART RCT.Southampton (UK): National Institute for Health and Care Research; 2023 Sep. Southampton (UK): National Institute for Health and Care Research; 2023 Sep. PMID: 37851847 Free Books & Documents. Review.
-
Design and rationale of the ATHENA study--A 12-month, multicentre, prospective study evaluating the outcomes of a de novo everolimus-based regimen in combination with reduced cyclosporine or tacrolimus versus a standard regimen in kidney transplant patients: study protocol for a randomised controlled trial.Trials. 2016 Feb 17;17:92. doi: 10.1186/s13063-016-1220-9. Trials. 2016. PMID: 26888217 Free PMC article. Clinical Trial.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.Health Technol Assess. 2005 May;9(21):1-179, iii-iv. doi: 10.3310/hta9210. Health Technol Assess. 2005. PMID: 15899149 Review.
Cited by
-
Optimized immunosuppression to prevent graft failure in renal transplant recipients with HLA antibodies (OuTSMART): a randomised controlled trial.EClinicalMedicine. 2023 Jan 12;56:101819. doi: 10.1016/j.eclinm.2022.101819. eCollection 2023 Feb. EClinicalMedicine. 2023. PMID: 36684392 Free PMC article.
-
Immune-related adverse event in the emergency department: methodology of the immune-related emergency disposition index (IrEDi).Emerg Cancer Care. 2024;3(1):1. doi: 10.1186/s44201-023-00023-y. Epub 2024 Jan 29. Emerg Cancer Care. 2024. PMID: 38725994 Free PMC article.
-
On the clinical relevance of using complete high-resolution HLA typing for an accurate interpretation of posttransplant immune-mediated graft outcomes.Front Immunol. 2022 Sep 29;13:924825. doi: 10.3389/fimmu.2022.924825. eCollection 2022. Front Immunol. 2022. PMID: 36248818 Free PMC article.
References
-
- Dorling A, Rebollo-Mesa I, Hilton R, Peacock JL, Vaughan R, Gardner L, et al. Can a combined screening/treatment programme prevent premature failure of renal transplants due to chronic rejection in patients with HLA antibodies: study protocol for the multicentre randomised controlled OuTSMART trial. Trials. 2014;15(1):30. doi: 10.1186/1745-6215-15-30. - DOI - PMC - PubMed
-
- Cytel Inc., Cambridge MA. East 6 (2016). Statistical software for the design, simulation and monitoring clinical trials. https://www.cytel.com/software-solutions/east/faq.
-
- Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009;87(10):1505–1513. doi: 10.1097/TP.0b013e3181a44206. - DOI - PubMed
-
- Dudley C, Pohanka E, Riad H, Dedochova J, Wijngaard P, Sutter C, et al. Mycophenolate mofetil substitution for cyclosporine A in renal transplant recipients with chronic progressive allograft dysfunction: The ‘Creeping Creatinine’ Study. Transplantation. 2005;79(4):466–475. doi: 10.1097/01.TP.0000151632.21551.00. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

