A current view of G protein-coupled receptor - mediated signaling in pulmonary hypertension: finding opportunities for therapeutic intervention
- PMID: 31380505
- PMCID: PMC6677404
- DOI: 10.20517/2574-1209.2018.44
A current view of G protein-coupled receptor - mediated signaling in pulmonary hypertension: finding opportunities for therapeutic intervention
Abstract
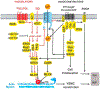
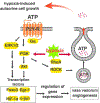
Pathological vascular remodeling is observed in various cardiovascular diseases including pulmonary hypertension (PH), a disease of unknown etiology that has been characterized by pulmonary artery vasoconstriction, right ventricular hypertrophy, vascular inflammation, and abnormal angiogenesis in pulmonary circulation. G protein-coupled receptors (GPCRs) are the largest family in the genome and widely expressed in cardiovascular system. They regulate all aspects of PH pathophysiology and represent therapeutic targets. We overview GPCRs function in vasoconstriction, vasodilation, vascular inflammation-driven remodeling and describe signaling cross talk between GPCR, inflammatory cytokines, and growth factors. Overall, the goal of this review is to emphasize the importance of GPCRs as critical signal transducers and targets for drug development in PH.
Keywords: GPCR; Pulmonary hypertension; intracellular signaling; vascular inflammation; vascular remodeling; vasoconstriction.
Conflict of interest statement
Conflicts of interest All authors declared that there are no conflicts of interest.
Figures

Similar articles
-
Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension.Mol Cell Endocrinol. 2015 Mar 5;403:46-56. doi: 10.1016/j.mce.2015.01.012. Epub 2015 Jan 13. Mol Cell Endocrinol. 2015. PMID: 25595485
-
Chronic iron overload induces vascular dysfunction in resistance pulmonary arteries associated with right ventricular remodeling in rats.Toxicol Lett. 2018 Oct 1;295:296-306. doi: 10.1016/j.toxlet.2018.07.010. Epub 2018 Jul 7. Toxicol Lett. 2018. PMID: 29990562
-
New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: importance of endothelial communication.Chest. 2015 Feb;147(2):529-537. doi: 10.1378/chest.14-0862. Chest. 2015. PMID: 25644906 Review.
-
Keeping the Balance Right: Regulator of G Protein Signaling 5 in Vascular Physiology and Pathology.Prog Mol Biol Transl Sci. 2015;133:93-121. doi: 10.1016/bs.pmbts.2015.02.003. Epub 2015 Apr 1. Prog Mol Biol Transl Sci. 2015. PMID: 26123304 Review.
-
Serotonin 2A (5-HT2A) Receptor Function: Ligand-Dependent Mechanisms and Pathways.In: Chattopadhyay A, editor. Serotonin Receptors in Neurobiology. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 6. In: Chattopadhyay A, editor. Serotonin Receptors in Neurobiology. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 6. PMID: 21204452 Free Books & Documents. Review.
Cited by
-
Vascular remodeling 2018: the updates.Vessel Plus. 2019;3:11. doi: 10.20517/2574-1209.2019.11. Epub 2019 Apr 17. Vessel Plus. 2019. PMID: 33981963 Free PMC article.
-
Mechanism of Beraprost Effects on Pulmonary Hypertension: Contribution of Cross-Binding to PGE2 Receptor 4 and Modulation of O2 Sensitive Voltage-Gated K+ Channels.Front Pharmacol. 2019 Jan 18;9:1518. doi: 10.3389/fphar.2018.01518. eCollection 2018. Front Pharmacol. 2019. PMID: 30713496 Free PMC article.
-
Clinical Effectiveness of a Combination of Black Elder Berries, Violet Herb, and Calendula Flowers in Chronic Obstructive Pulmonary Disease: The Results of a Double-Blinded Placebo-Controlled Study.Biology (Basel). 2020 Apr 22;9(4):83. doi: 10.3390/biology9040083. Biology (Basel). 2020. PMID: 32331341 Free PMC article.
-
Pharmacological Gq inhibition induces strong pulmonary vasorelaxation and reverses pulmonary hypertension.EMBO Mol Med. 2024 Aug;16(8):1930-1956. doi: 10.1038/s44321-024-00096-0. Epub 2024 Jul 8. EMBO Mol Med. 2024. PMID: 38977926 Free PMC article.
-
Characterization of the Impacts of Living at High Altitude in Taif: Oxidative Stress Biomarker Alterations and Immunohistochemical Changes.Curr Issues Mol Biol. 2022 Apr 9;44(4):1610-1625. doi: 10.3390/cimb44040110. Curr Issues Mol Biol. 2022. PMID: 35723368 Free PMC article.
References
-
- Joppi R, Gerardi C, Bertele V, Garattini S. A disease looking for innovative drugs: the case of pulmonary arterial hypertension. Eur J Intern Med 2018;55:47–51. - PubMed
-
- Thomsen W, Frazer J, Unett D. Functional assays for screening GPCR targets. Curr Opin Biotechnol 2005;16:655–65. - PubMed
-
- Offermanns S, Simon MI. Organization of transmembrane signalling by heterotrimeric G proteins. Cancer Surv 1996;27:177–98. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
