Through the Looking Glass: In Vitro Models for Inhalation Toxicology and Interindividual Variability in the Airway
- PMID: 31380467
- PMCID: PMC6067645
- DOI: 10.1089/aivt.2018.0002
Through the Looking Glass: In Vitro Models for Inhalation Toxicology and Interindividual Variability in the Airway
Abstract
With 7 million deaths reported annually from air pollution alone, it is evident that adverse effects of inhaled toxicant exposures remain a major public health concern in the 21st century. Assessment and characterization of the impacts of air pollutants on human health stems from epidemiological and clinical studies, which have linked both outdoor and indoor air contaminant exposure to adverse pulmonary and cardiovascular health outcomes. Studies in animal models support epidemiological findings and have been critical in identifying systemic effects of environmental chemicals on cognitive abilities, liver disease, and metabolic dysfunction following inhalation exposure. Likewise, traditional monoculture systems have aided in identifying biomarkers of susceptibility to inhaled toxicants and served as a screening platform for safety assessment of pulmonary toxicants. Despite their contributions, in vivo and classic in vitro models have not been able to accurately represent the heterogeneity of the human population and account for interindividual variability in response to inhaled toxicants and susceptibility to the adverse health effects. Development of new technologies that can investigate genetic predisposition, are cost and time efficient, and are ethically sound, will enhance elucidation of mechanisms of inhalation toxicity, and aid in the development of novel pharmaceuticals and/or safety evaluation. This review will describe the classic and novel cell-based inhalation toxicity models and how these emerging technologies can be incorporated into regulatory or nonregulatory testing to address interindividual variability and improve overall human health.
Keywords: in vitro; inhalation; interindividual variability; lung; three-dimensional model; toxicity.
Conflict of interest statement
No competing financial interests exist.
Figures

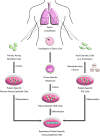
Similar articles
-
Effects of concentrated ambient particles on normal and hypersecretory airways in rats.Res Rep Health Eff Inst. 2004 Aug;(120):1-68; discussion 69-79. Res Rep Health Eff Inst. 2004. PMID: 15543855
-
Evaluating heterogeneity in indoor and outdoor air pollution using land-use regression and constrained factor analysis.Res Rep Health Eff Inst. 2010 Dec;(152):5-80; discussion 81-91. Res Rep Health Eff Inst. 2010. PMID: 21409949
-
Emerging Technologies for In Vitro Inhalation Toxicology.Adv Healthc Mater. 2021 Sep;10(18):e2100633. doi: 10.1002/adhm.202100633. Epub 2021 Jul 22. Adv Healthc Mater. 2021. PMID: 34292676 Free PMC article. Review.
-
Inhalation toxicology.EXS. 2010;100:461-88. doi: 10.1007/978-3-7643-8338-1_13. EXS. 2010. PMID: 20358692 Review.
-
Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions.Toxicol In Vitro. 2018 Mar;47:137-146. doi: 10.1016/j.tiv.2017.11.005. Epub 2017 Nov 15. Toxicol In Vitro. 2018. PMID: 29155131 Review.
Cited by
-
Host and Pathogen Communication in the Respiratory Tract: Mechanisms and Models of a Complex Signaling Microenvironment.Front Med (Lausanne). 2020 Sep 10;7:537. doi: 10.3389/fmed.2020.00537. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33015094 Free PMC article. Review.
-
In Vitro Systems for Toxicity Evaluation of Microbial Volatile Organic Compounds on Humans: Current Status and Trends.J Fungi (Basel). 2022 Jan 13;8(1):75. doi: 10.3390/jof8010075. J Fungi (Basel). 2022. PMID: 35050015 Free PMC article. Review.
-
Liquid application dosing alters the physiology of air-liquid interface (ALI) primary human bronchial epithelial cell/lung fibroblast co-cultures and in vitro testing relevant endpoints.Front Toxicol. 2024 Jan 23;5:1264331. doi: 10.3389/ftox.2023.1264331. eCollection 2023. Front Toxicol. 2024. PMID: 38464699 Free PMC article.
-
Advancing understanding of human variability through toxicokinetic modeling, in vitro-in vivo extrapolation, and new approach methodologies.Hum Genomics. 2024 Nov 21;18(1):129. doi: 10.1186/s40246-024-00691-9. Hum Genomics. 2024. PMID: 39574200 Free PMC article. Review.
-
Advanced human-relevant in vitro pulmonary platforms for respiratory therapeutics.Adv Drug Deliv Rev. 2021 Sep;176:113901. doi: 10.1016/j.addr.2021.113901. Epub 2021 Jul 29. Adv Drug Deliv Rev. 2021. PMID: 34331989 Free PMC article. Review.
References
-
- Schechtman LM. Implementation of the 3Rs (refinement, reduction, and replacement): Validation and regulatory acceptance considerations for alternative toxicological test methods. ILAR J 2002:43 Suppl;S85–S94 - PubMed
-
- Belliveau ME. The drive for a safer chemicals policy in the United States. New Solut 2011:21;359–386 - PubMed
-
- Impinen A, Nygaard UC, Lodrup Carlsen KC, et al. . Prenatal exposure to perfluoralkyl substances (PFASs) associated with respiratory tract infections but not allergy- and asthma-related health outcomes in childhood. Environ Res 2018:160;518–523 - PubMed
-
- McCullough SD, Duncan KE, Swanton SM, et al. . Ozone induces a proinflammatory response in primary human bronchial epithelial cells through mitogen-activated protein kinase activation without nuclear factor-κB activation. Am J Respir Cell Mol Biol 2014:51;426–435 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
