Pathogenesis of Important Virulence Factors of Porphyromonas gingivalis via Toll-Like Receptors
- PMID: 31380305
- PMCID: PMC6657652
- DOI: 10.3389/fcimb.2019.00262
Pathogenesis of Important Virulence Factors of Porphyromonas gingivalis via Toll-Like Receptors
Abstract
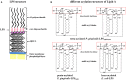
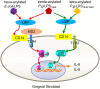
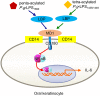
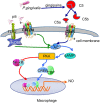
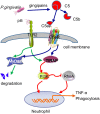
Periodontitis is a common intraoral infection and is inextricably linked to systemic diseases. Recently, the regulation between host immunologic response and periodontal pathogens has become a hotspot to explain the mechanism of periodontitis and related systemic diseases. Since Porphyromonas gingivalis (P. gingivalis) was proved as critical periodontal pathogen above all, researches focusing on the mechanism of its virulence factors have received extensive attention. Studies have shown that in the development of periodontitis, in addition to the direct release of virulent factors by periodontal pathogens to destroy periodontal tissues, over-low or over-high intrinsic immune and inflammatory response mediated by Toll-like receptors (TLRs) can lead to more lasting destruction of periodontal tissues. It is very necessary to sort out how various cytopathic factors of P. gingivalis mediate inflammation and immune responses between the host through TLRs so as to help precisely prevent, diagnose, and treat periodontitis in clinic. This review summarizes the role of three most widely studied pathogenic factors produced by P. gingivalis (lipopolysaccharide, gingipains, pili) and their interactions with TLRs at the cellular and molecular level in the progress of periodontitis.
Keywords: Porphyromonas gingivalis; Toll-like receptors; fimbriae; gingipains; lipopolysaccharide; periodontitis; virulence factor.
Figures

Similar articles
-
Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen.FEMS Microbiol Lett. 2012 Aug;333(1):1-9. doi: 10.1111/j.1574-6968.2012.02579.x. Epub 2012 May 28. FEMS Microbiol Lett. 2012. PMID: 22530835 Review.
-
A quantitative framework reveals traditional laboratory growth is a highly accurate model of human oral infection.Proc Natl Acad Sci U S A. 2022 Jan 11;119(2):e2116637119. doi: 10.1073/pnas.2116637119. Proc Natl Acad Sci U S A. 2022. PMID: 34992142 Free PMC article.
-
The role of gingipains in the pathogenesis of periodontal disease.J Periodontol. 2003 Jan;74(1):111-8. doi: 10.1902/jop.2003.74.1.111. J Periodontol. 2003. PMID: 12593605 Review.
-
Type IX secretion system is pivotal for expression of gingipain-associated virulence of Porphyromonas gingivalis.Mol Oral Microbiol. 2019 Dec;34(6):237-244. doi: 10.1111/omi.12268. Epub 2019 Oct 27. Mol Oral Microbiol. 2019. PMID: 31432617
-
Molecular Strategies Underlying Porphyromonas gingivalis Virulence.J Mol Biol. 2021 Apr 2;433(7):166836. doi: 10.1016/j.jmb.2021.166836. Epub 2021 Feb 1. J Mol Biol. 2021. PMID: 33539891 Review.
Cited by
-
A Porphyromonas gingivalis hypothetical protein controlled by the type I-C CRISPR-Cas system is a novel adhesin important in virulence.mSystems. 2024 Mar 19;9(3):e0123123. doi: 10.1128/msystems.01231-23. Epub 2024 Feb 7. mSystems. 2024. PMID: 38323815 Free PMC article.
-
The relationship between periodontal status and rheumatoid arthritis - systematic review.Reumatologia. 2020;58(4):236-242. doi: 10.5114/reum.2020.98436. Epub 2020 Aug 31. Reumatologia. 2020. PMID: 32921831 Free PMC article. Review.
-
Coniferyl Aldehyde Inhibits the Inflammatory Effects of Leptomeningeal Cells by Suppressing the JAK2 Signaling.Biomed Res Int. 2020 Sep 14;2020:4616308. doi: 10.1155/2020/4616308. eCollection 2020. Biomed Res Int. 2020. PMID: 33015166 Free PMC article.
-
High glucose condition aggravates inflammatory response induced by Porphyromonas gingivalis in THP-1 macrophages via autophagy inhibition.BMC Immunol. 2024 Oct 17;25(1):69. doi: 10.1186/s12865-024-00655-7. BMC Immunol. 2024. PMID: 39415131 Free PMC article.
-
Modulatory Mechanisms of Pathogenicity in Porphyromonas gingivalis and Other Periodontal Pathobionts.Microorganisms. 2022 Dec 21;11(1):15. doi: 10.3390/microorganisms11010015. Microorganisms. 2022. PMID: 36677306 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

