Systemic Juvenile Idiopathic Arthritis-Associated Lung Disease: Characterization and Risk Factors
- PMID: 31379071
- PMCID: PMC6817389
- DOI: 10.1002/art.41073
Systemic Juvenile Idiopathic Arthritis-Associated Lung Disease: Characterization and Risk Factors
Abstract
Objective: Systemic juvenile idiopathic arthritis (JIA) is associated with a recently recognized, albeit poorly defined and characterized, lung disease (LD). The objective of this study was to describe the clinical characteristics, risk factors, and histopathologic and immunologic features of this novel inflammatory LD associated with systemic JIA (designated SJIA-LD).
Methods: Clinical data collected since 2010 were abstracted from the medical records of patients with systemic JIA from the Cincinnati Children's Hospital Medical Center. Epidemiologic, cellular, biochemical, genomic, and transcriptional profiling analyses were performed.
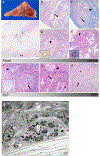
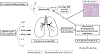
Results: Eighteen patients with SJIA-LD were identified. Radiographic findings included diffuse ground-glass opacities, subpleural reticulation, interlobular septal thickening, and lymphadenopathy. Pathologic findings included patchy, but extensive, lymphoplasmacytic infiltrates and mixed features of pulmonary alveolar proteinosis (PAP) and endogenous lipoid pneumonia. Compared to systemic JIA patients without LD, those with SJIA-LD were younger at the diagnosis of systemic JIA (odds ratio [OR] 6.5, P = 0.007), more often had prior episodes of macrophage activation syndrome (MAS) (OR 14.5, P < 0.001), had a greater frequency of adverse reactions to biologic therapy (OR 13.6, P < 0.001), and had higher serum levels of interleukin-18 (IL-18) (median 27,612 pg/ml versus 5,413 pg/ml; P = 0.047). Patients with SJIA-LD lacked genetic, serologic, or functional evidence of granulocyte-macrophage colony-stimulating factor pathway dysfunction, a feature that is typical of familial or autoimmune PAP. Moreover, bronchoalveolar lavage (BAL) fluid from patients with SJIA-LD rarely demonstrated proteinaceous material and had less lipid-laden macrophages than that seen in patients with primary PAP (mean 10.5% in patients with SJIA-LD versus 66.1% in patients with primary PAP; P < 0.001). BAL fluid from patients with SJIA-LD contained elevated levels of IL-18 and the interferon-γ-induced chemokines CXCL9 and CXCL10. Transcriptional profiling of the lung tissue from patients with SJIA-LD identified up-regulated type II interferon and T cell activation networks. This signature was also present in SJIA-LD human lung tissue sections that lacked substantial histopathologic findings, suggesting that this activation signature may precede and drive the lung pathology in SJIA-LD.
Conclusion: Pulmonary disease is increasingly detected in children with systemic JIA, particularly in association with MAS. This entity has distinct clinical and immunologic features and represents an uncharacterized inflammatory LD.
© 2019, American College of Rheumatology.
Conflict of interest statement
All other authors declare no conflicts of interest.
Figures




Similar articles
-
Identification of Distinct Inflammatory Programs and Biomarkers in Systemic Juvenile Idiopathic Arthritis and Related Lung Disease by Serum Proteome Analysis.Arthritis Rheumatol. 2022 Jul;74(7):1271-1283. doi: 10.1002/art.42099. Epub 2022 May 31. Arthritis Rheumatol. 2022. PMID: 35189047 Free PMC article.
-
Emergent high fatality lung disease in systemic juvenile arthritis.Ann Rheum Dis. 2019 Dec;78(12):1722-1731. doi: 10.1136/annrheumdis-2019-216040. Epub 2019 Sep 27. Ann Rheum Dis. 2019. PMID: 31562126 Free PMC article.
-
Adenosine deaminase 2 as a biomarker of macrophage activation syndrome in systemic juvenile idiopathic arthritis.Ann Rheum Dis. 2020 Feb;79(2):225-231. doi: 10.1136/annrheumdis-2019-216030. Epub 2019 Nov 9. Ann Rheum Dis. 2020. PMID: 31707357 Free PMC article.
-
Interfering with interferons: targeting the JAK-STAT pathway in complications of systemic juvenile idiopathic arthritis (SJIA).Rheumatology (Oxford). 2022 Mar 2;61(3):926-935. doi: 10.1093/rheumatology/keab673. Rheumatology (Oxford). 2022. PMID: 34459891 Free PMC article. Review.
-
Lung Involvement in Systemic Juvenile Idiopathic Arthritis: A Narrative Review.Diagnostics (Basel). 2022 Dec 8;12(12):3095. doi: 10.3390/diagnostics12123095. Diagnostics (Basel). 2022. PMID: 36553101 Free PMC article. Review.
Cited by
-
Total metabolic lesion volume of lymph nodes measured by 18F-FDG PET/CT: a new predictor of macrophage activation syndrome in adult-onset Still's disease.Arthritis Res Ther. 2021 Mar 30;23(1):97. doi: 10.1186/s13075-021-02482-2. Arthritis Res Ther. 2021. PMID: 33785060 Free PMC article.
-
Parenchymal lung disease in adult onset Still's disease: an emergent marker of disease severity-characterisation and predictive factors from Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale (GIRRCS) cohort of patients.Arthritis Res Ther. 2020 Jun 22;22(1):151. doi: 10.1186/s13075-020-02245-5. Arthritis Res Ther. 2020. PMID: 32571407 Free PMC article.
-
Clinicopathologic Conference: A Four-Year-Old Child With Digital Clubbing.Arthritis Care Res (Hoboken). 2021 Oct;73(10):1379-1386. doi: 10.1002/acr.24428. Arthritis Care Res (Hoboken). 2021. PMID: 32813330 Free PMC article. No abstract available.
-
Why judiciously timed anti-IL 6 therapy may be of benefit in severe COVID-19 infection.Autoimmun Rev. 2020 Jul;19(7):102563. doi: 10.1016/j.autrev.2020.102563. Epub 2020 May 5. Autoimmun Rev. 2020. PMID: 32380318 Free PMC article. No abstract available.
-
Cytokine Storm Syndrome Associated with Systemic Juvenile Idiopathic Arthritis.Adv Exp Med Biol. 2024;1448:323-353. doi: 10.1007/978-3-031-59815-9_23. Adv Exp Med Biol. 2024. PMID: 39117825 Review.
References
-
- Woo P Systemic juvenile idiopathic arthritis: diagnosis, management, and outcome. Nat Clin Pract Rheumatol 2006;2:28–34. Available at: http://www.nature.com/articles/ncprheum0084. Accessed February 15, 2019. - PubMed
-
- Mellins ED, Macaubas C, Grom AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol 2011;7:416–426. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21647204. - PMC - PubMed
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14760812. Accessed July 2, 2015. - PubMed
-
- Lomater C, Gerloni V, Gattinara M, Mazzotti J, Cimaz R, Fantini F. Systemic onset juvenile idiopathic arthritis: a retrospective study of 80 consecutive patients followed for 10 years. J Rheumatol 2000;27:491–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10685819. Accessed October 5, 2015. - PubMed
-
- Lang BA, Schneider R, Reilly BJ, Silverman ED, Laxer RM. Radiologic features of systemic onset juvenile rheumatoid arthritis. J Rheumatol 1995;22:168–73. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7699666. Accessed February 15, 2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

