Hydrogen peroxide release by bacteria suppresses inflammasome-dependent innate immunity
- PMID: 31375698
- PMCID: PMC6677825
- DOI: 10.1038/s41467-019-11169-x
Hydrogen peroxide release by bacteria suppresses inflammasome-dependent innate immunity
Abstract
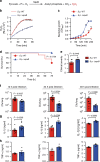
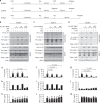
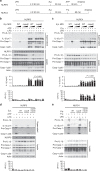
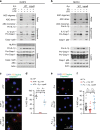
Hydrogen peroxide (H2O2) has a major function in host-microbial interactions. Although most studies have focused on the endogenous H2O2 produced by immune cells to kill microbes, bacteria can also produce H2O2. How microbial H2O2 influences the dynamics of host-microbial interactions is unclear. Here we show that H2O2 released by Streptococcus pneumoniae inhibits inflammasomes, key components of the innate immune system, contributing to the pathogen colonization of the host. We also show that the oral commensal H2O2-producing bacteria Streptococcus oralis can block inflammasome activation. This study uncovers an unexpected role of H2O2 in immune suppression and demonstrates how, through this mechanism, bacteria might restrain the immune system to co-exist with the host.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Microarray analysis of macrophage response to infection with Streptococcus oralis reveals the immunosuppressive effect of hydrogen peroxide.Biochem Biophys Res Commun. 2017 Apr 1;485(2):461-467. doi: 10.1016/j.bbrc.2017.02.048. Epub 2017 Feb 13. Biochem Biophys Res Commun. 2017. PMID: 28202416
-
Activation of ASC Inflammasome Driven by Toll-Like Receptor 4 Contributes to Host Immunity against Rickettsial Infection.Infect Immun. 2020 Mar 23;88(4):e00886-19. doi: 10.1128/IAI.00886-19. Print 2020 Mar 23. Infect Immun. 2020. PMID: 32014896 Free PMC article.
-
Hydrogen peroxide produced by oral Streptococci induces macrophage cell death.PLoS One. 2013 May 3;8(5):e62563. doi: 10.1371/journal.pone.0062563. Print 2013. PLoS One. 2013. PMID: 23658745 Free PMC article.
-
Inflammasomes in inflammatory bowel disease pathogenesis.Curr Opin Gastroenterol. 2013 Jul;29(4):363-9. doi: 10.1097/MOG.0b013e32836157a4. Curr Opin Gastroenterol. 2013. PMID: 23689522 Review.
-
The NLRP1 inflammasomes.Immunol Rev. 2015 May;265(1):22-34. doi: 10.1111/imr.12283. Immunol Rev. 2015. PMID: 25879281 Review.
Cited by
-
Host Cell Oxidative Stress Promotes Intracellular Fluoroquinolone Persisters of Streptococcus pneumoniae.Microbiol Spectr. 2022 Dec 21;10(6):e0436422. doi: 10.1128/spectrum.04364-22. Epub 2022 Nov 29. Microbiol Spectr. 2022. PMID: 36445159 Free PMC article.
-
On-Demand Synthesis of Antiseptics at the Site of Infection for Treatment of Otitis Media.Nano Today. 2022 Dec;47:101672. doi: 10.1016/j.nantod.2022.101672. Epub 2022 Nov 10. Nano Today. 2022. PMID: 36968792 Free PMC article.
-
Heme Peroxidases at Unperturbed and Inflamed Mucous Surfaces.Antioxidants (Basel). 2021 Nov 12;10(11):1805. doi: 10.3390/antiox10111805. Antioxidants (Basel). 2021. PMID: 34829676 Free PMC article. Review.
-
Oral streptococci subvert the host innate immune response through hydrogen peroxide.Sci Rep. 2022 Jan 13;12(1):656. doi: 10.1038/s41598-021-04562-4. Sci Rep. 2022. PMID: 35027607 Free PMC article.
-
Oral streptococci: modulators of health and disease.Front Cell Infect Microbiol. 2024 Feb 22;14:1357631. doi: 10.3389/fcimb.2024.1357631. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38456080 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

