Rapid Elimination of Broadly Neutralizing Antibodies Correlates with Treatment Failure in the Acute Phase of Simian-Human Immunodeficiency Virus Infection
- PMID: 31375583
- PMCID: PMC6798097
- DOI: 10.1128/JVI.01077-19
Rapid Elimination of Broadly Neutralizing Antibodies Correlates with Treatment Failure in the Acute Phase of Simian-Human Immunodeficiency Virus Infection
Abstract
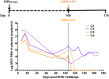
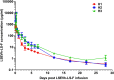
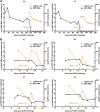
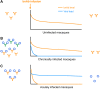
Early human immunodeficiency virus type 1 (HIV-1) treatment during the acute period of infection can significantly limit the seeding of viral reservoirs and modify the course of disease. However, while a number of HIV-1 broadly neutralizing antibodies (bnAbs) have demonstrated remarkable efficacy as prophylaxis in macaques chronically infected with simian-human immunodeficiency virus (SHIV), intriguingly, their inhibitory effects were largely attenuated in the acute period of SHIV infection. To investigate the mechanism for the disparate performance of bnAbs in different periods of SHIV infection, we used LSEVh-LS-F, a bispecific bnAb targeting the CD4 binding site and CD4-induced epitopes, as a representative bnAb and assessed its potential therapeutic benefit in controlling virus replication in acutely or chronically SHIV-infected macaques. We found that a single infusion of LSEVh-LS-F resulted in rapid decline of plasma viral loads to undetectable levels without emergence of viral resistance in the chronically infected macaques. In contrast, the inhibitory effect was robust but transient in the acutely infected macaques, despite the fact that all macaques had comparable plasma viral loads initially. Infusing multiple doses of LSEVh-LS-F did not extend its inhibitory duration. Furthermore, the pharmacokinetics of the infused LSEVh-LS-F in the acutely SHIV-infected macaques significantly differed from that in the uninfected or chronically infected macaques. Host SHIV-specific immune responses may play a role in the viremia-dependent pharmacokinetics. Our results highlight the correlation between the fast clearance of infused bnAbs and the treatment failure in the acute period of SHIV infection and may have important implications for the therapeutic use of bnAbs to treat acute HIV infections.IMPORTANCE Currently, there is no bnAb-based monotherapy that has been reported to clear the virus in the acute SHIV infection period. Since early HIV treatment is considered critical to restricting the establishment of viral reservoirs, investigation into the mechanism for treatment failure in acutely infected macaques would be important for the therapeutic use of bnAbs and eventually towards the functional cure of HIV/AIDS. Here we report the comparative study of the therapeutic efficacy of a bnAb in acutely and chronically SHIV-infected macaques. This study revealed the correlation between the fast clearance of infused bnAbs and treatment failure during the acute period of infection.
Keywords: HIV-1; acute SHIV infection; broadly neutralizing antibodies.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
Potent In Vivo NK Cell-Mediated Elimination of HIV-1-Infected Cells Mobilized by a gp120-Bispecific and Hexavalent Broadly Neutralizing Fusion Protein.J Virol. 2017 Sep 27;91(20):e00937-17. doi: 10.1128/JVI.00937-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28794022 Free PMC article.
-
Development of Broadly Neutralizing Antibodies and Their Mapping by Monomeric gp120 in Human Immunodeficiency Virus Type 1-Infected Humans and Simian-Human Immunodeficiency Virus SHIVSF162P3N-Infected Macaques.J Virol. 2016 Mar 28;90(8):4017-4031. doi: 10.1128/JVI.02898-15. Print 2016 Apr. J Virol. 2016. PMID: 26842476 Free PMC article.
-
Virological Control by the CD4-Binding Site Antibody N6 in Simian-Human Immunodeficiency Virus-Infected Rhesus Monkeys.J Virol. 2017 Jul 27;91(16):e00498-17. doi: 10.1128/JVI.00498-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28539448 Free PMC article.
-
Vaccinal effect of HIV-1 antibody therapy.Curr Opin HIV AIDS. 2019 Jul;14(4):325-333. doi: 10.1097/COH.0000000000000555. Curr Opin HIV AIDS. 2019. PMID: 30973419 Review.
-
Fc receptor-mediated antiviral antibodies.Curr Opin HIV AIDS. 2009 Sep;4(5):388-93. doi: 10.1097/COH.0b013e32832f0a89. Curr Opin HIV AIDS. 2009. PMID: 20048702 Free PMC article. Review.
Cited by
-
CRISPR-Cas9 Mediated Exonic Disruption for HIV-1 Elimination.EBioMedicine. 2021 Nov;73:103678. doi: 10.1016/j.ebiom.2021.103678. Epub 2021 Nov 10. EBioMedicine. 2021. PMID: 34774454 Free PMC article.
-
Pathways towards human immunodeficiency virus elimination.EBioMedicine. 2020 Mar;53:102667. doi: 10.1016/j.ebiom.2020.102667. Epub 2020 Feb 27. EBioMedicine. 2020. PMID: 32114397 Free PMC article. Review.
-
Engineering a Novel Antibody-Peptide Bispecific Fusion Protein Against MERS-CoV.Antibodies (Basel). 2019 Nov 4;8(4):53. doi: 10.3390/antib8040053. Antibodies (Basel). 2019. PMID: 31690009 Free PMC article.
-
Humanized Mice for Studies of HIV-1 Persistence and Elimination.Pathogens. 2023 Jun 27;12(7):879. doi: 10.3390/pathogens12070879. Pathogens. 2023. PMID: 37513726 Free PMC article. Review.
-
Rapid identification of a human antibody with high prophylactic and therapeutic efficacy in three animal models of SARS-CoV-2 infection.Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29832-29838. doi: 10.1073/pnas.2010197117. Epub 2020 Nov 2. Proc Natl Acad Sci U S A. 2020. PMID: 33139569 Free PMC article.
References
-
- Davey RT Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS, Lane HC. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 96:15109–15114. doi:10.1073/pnas.96.26.15109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

