Murine Cytomegalovirus Infection of Melanoma Lesions Delays Tumor Growth by Recruiting and Repolarizing Monocytic Phagocytes in the Tumor
- PMID: 31375579
- PMCID: PMC6798091
- DOI: 10.1128/JVI.00533-19
Murine Cytomegalovirus Infection of Melanoma Lesions Delays Tumor Growth by Recruiting and Repolarizing Monocytic Phagocytes in the Tumor
Abstract
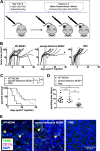
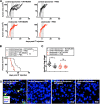
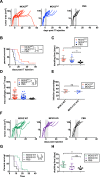
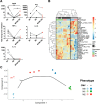
Cytomegalovirus (CMV) is a ubiquitous betaherpesvirus that infects many different cell types. Human CMV (HCMV) has been found in several solid tumors, and it has been hypothesized that it may promote cellular transformation or exacerbate tumor growth. Paradoxically, in some experimental situations, murine CMV (MCMV) infection delays tumor growth. We previously showed that wild-type MCMV delayed the growth of poorly immunogenic B16 melanomas via an undefined mechanism. Here, we show that MCMV delayed the growth of these immunologically "cold" tumors by recruiting and modulating tumor-associated macrophages. Depletion of monocytic phagocytes with clodronate completely prevented MCMV from delaying tumor growth. Mechanistically, our data suggest that MCMV recruits new macrophages to the tumor via the virus-encoded chemokine MCK2, and viruses lacking this chemokine were unable to delay tumor growth. Moreover, MCMV infection of macrophages drove them toward a proinflammatory (M1)-like state. Importantly, adaptive immune responses were also necessary for MCMV to delay tumor growth as the effect was substantially blunted in Rag-deficient animals. However, viral spread was not needed and a spread-defective MCMV strain was equally effective. In most mice, the antitumor effect of MCMV was transient. Although the recruited macrophages persisted, tumor regrowth correlated with a loss of viral activity in the tumor. However, an additional round of MCMV infection further delayed tumor growth, suggesting that tumor growth delay was dependent on active viral infection. Together, our results suggest that MCMV infection delayed the growth of an immunologically cold tumor by recruiting and modulating macrophages in order to promote anti-tumor immune responses.IMPORTANCE Cytomegalovirus (CMV) is an exciting new platform for vaccines and cancer therapy. Although CMV may delay tumor growth in some settings, there is also evidence that CMV may promote cancer development and progression. Thus, defining the impact of CMV on tumors is critical. Using a mouse model of melanoma, we previously found that murine CMV (MCMV) delayed tumor growth and activated tumor-specific immunity although the mechanism was unclear. We now show that MCMV delayed tumor growth through a mechanism that required monocytic phagocytes and a viral chemokine that recruited macrophages to the tumor. Furthermore, MCMV infection altered the functional state of macrophages. Although the effects of MCMV on tumor growth were transient, we found that repeated MCMV injections sustained the antitumor effect, suggesting that active viral infection was needed. Thus, MCMV altered tumor growth by actively recruiting macrophages to the tumor, where they were modulated to promote antitumor immunity.
Keywords: cytomegalovirus; immunomodulation; immunotherapy; macrophages; melanoma.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
STING Sensing of Murine Cytomegalovirus Alters the Tumor Microenvironment to Promote Antitumor Immunity.J Immunol. 2020 Jun 1;204(11):2961-2972. doi: 10.4049/jimmunol.1901136. Epub 2020 Apr 13. J Immunol. 2020. PMID: 32284333 Free PMC article.
-
Murine Cytomegalovirus Deubiquitinase Regulates Viral Chemokine Levels To Control Inflammation and Pathogenesis.mBio. 2017 Jan 17;8(1):e01864-16. doi: 10.1128/mBio.01864-16. mBio. 2017. PMID: 28096485 Free PMC article.
-
The Human Cytomegalovirus Chemokine vCXCL-1 Modulates Normal Dissemination Kinetics of Murine Cytomegalovirus In Vivo.mBio. 2019 Jun 25;10(3):e01289-19. doi: 10.1128/mBio.01289-19. mBio. 2019. PMID: 31239384 Free PMC article.
-
Intratumoral infection by CMV may change the tumor environment by directly interacting with tumor-associated macrophages to promote cancer immunity.Hum Vaccin Immunother. 2017 Aug 3;13(8):1778-1785. doi: 10.1080/21645515.2017.1331795. Epub 2017 Jun 12. Hum Vaccin Immunother. 2017. PMID: 28604162 Free PMC article. Review.
-
Novel therapeutic strategies against cytomegalovirus infection.Nat Immun. 1995 Sep;14(5-6):250-61. Nat Immun. 1995. PMID: 8933819 Review.
Cited by
-
Dissecting the cytomegalovirus CC chemokine: Chemokine activity and gHgLchemokine-dependent cell tropism are independent players in CMV infection.PLoS Pathog. 2023 Dec 8;19(12):e1011793. doi: 10.1371/journal.ppat.1011793. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38064525 Free PMC article.
-
A Review of Murine Cytomegalovirus as a Model for Human Cytomegalovirus Disease-Do Mice Lie?Int J Mol Sci. 2020 Dec 28;22(1):214. doi: 10.3390/ijms22010214. Int J Mol Sci. 2020. PMID: 33379272 Free PMC article. Review.
-
Hematopoietic cell-mediated dissemination of murine cytomegalovirus is regulated by NK cells and immune evasion.PLoS Pathog. 2021 Jan 28;17(1):e1009255. doi: 10.1371/journal.ppat.1009255. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33508041 Free PMC article.
-
STING Sensing of Murine Cytomegalovirus Alters the Tumor Microenvironment to Promote Antitumor Immunity.J Immunol. 2020 Jun 1;204(11):2961-2972. doi: 10.4049/jimmunol.1901136. Epub 2020 Apr 13. J Immunol. 2020. PMID: 32284333 Free PMC article.
-
The convergence of tumor suppressors on the type I interferon pathway in clear cell renal cell carcinoma and its therapeutic implications.Am J Physiol Cell Physiol. 2022 Nov 1;323(5):C1417-C1429. doi: 10.1152/ajpcell.00255.2022. Epub 2022 Sep 26. Am J Physiol Cell Physiol. 2022. PMID: 36154696 Free PMC article. Review.
References
-
- Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Müller CA, Pircher H, Pawelec G. 2003. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1). Exp Gerontol 38:911–920. doi:10.1016/S0531-5565(03)00134-7. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

