Autophagy activation promotes clearance of α-synuclein inclusions in fibril-seeded human neural cells
- PMID: 31375560
- PMCID: PMC6768637
- DOI: 10.1074/jbc.RA119.008733
Autophagy activation promotes clearance of α-synuclein inclusions in fibril-seeded human neural cells
Abstract
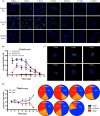
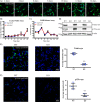
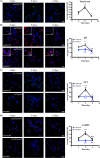
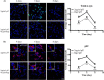
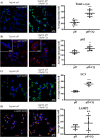
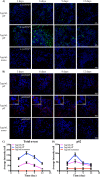
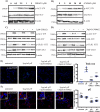
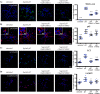
There is much interest in delineating the mechanisms by which the α-synuclein protein accumulates in brains of individuals with Parkinson's disease (PD). Preclinical studies with rodent and primate models have indicated that fibrillar forms of α-synuclein can initiate the propagation of endogenous α-synuclein pathology. However, the underlying mechanisms by which α-synuclein fibrils seed pathology remain unclear. To investigate this further, we have used exogenous fibrillar α-synuclein to seed endogenous α-synuclein pathology in human neuronal cell lines, including primary human neurons differentiated from induced pluripotent stem cells. Fluorescence microscopy and immunoblot analyses were used to monitor levels of α-synuclein and key autophagy/lysosomal proteins over time in the exogenous α-synuclein fibril-treated neurons. We observed that temporal changes in the accumulation of cytoplasmic α-synuclein inclusions were associated with changes in the key autophagy/lysosomal markers. Of note, chloroquine-mediated blockade of autophagy increased accumulation of α-synuclein inclusions, and rapamycin-induced activation of autophagy, or use of 5'-AMP-activated protein kinase (AMPK) agonists, promoted the clearance of fibril-mediated α-synuclein pathology. These results suggest a key role for autophagy in clearing fibrillar α-synuclein pathologies in human neuronal cells. We propose that our findings may help inform the development of human neural cell models for screening of potential therapeutic compounds for PD or for providing insight into the mechanisms of α-synuclein propagation. Our results further add to existing evidence that AMPK activation may be a therapeutic option for managing PD.
Keywords: AMP-activated kinase (AMPK); Lewy bodies; Parkinson disease; autophagy; neurodegeneration; neuron; p62 (sequestosome 1 (SQSTM1)); protein degradation; protein fibril; α-synuclein (α-synuclein).
© 2019 Gao et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
p62/SQSTM1-dependent autophagy of Lewy body-like α-synuclein inclusions.PLoS One. 2012;7(12):e52868. doi: 10.1371/journal.pone.0052868. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300799 Free PMC article.
-
Ubiquitination of alpha-synuclein and autophagy in Parkinson's disease.Autophagy. 2008 Apr;4(3):372-4. doi: 10.4161/auto.5604. Epub 2008 Jan 18. Autophagy. 2008. PMID: 18216494 Review.
-
Autophagy modulates SNCA/α-synuclein release, thereby generating a hostile microenvironment.Autophagy. 2014;10(12):2171-92. doi: 10.4161/auto.36436. Autophagy. 2014. PMID: 25484190 Free PMC article.
-
Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinson's disease.Cell Death Dis. 2013 Mar 14;4(3):e545. doi: 10.1038/cddis.2013.73. Cell Death Dis. 2013. PMID: 23492776 Free PMC article.
-
Alpha-synuclein, autophagy-lysosomal pathway, and Lewy bodies: Mutations, propagation, aggregation, and the formation of inclusions.J Biol Chem. 2024 Oct;300(10):107742. doi: 10.1016/j.jbc.2024.107742. Epub 2024 Sep 2. J Biol Chem. 2024. PMID: 39233232 Free PMC article. Review.
Cited by
-
CRISPR and iPSCs: Recent Developments and Future Perspectives in Neurodegenerative Disease Modelling, Research, and Therapeutics.Neurotox Res. 2022 Oct;40(5):1597-1623. doi: 10.1007/s12640-022-00564-w. Epub 2022 Aug 31. Neurotox Res. 2022. PMID: 36044181 Free PMC article. Review.
-
Pathogenesis of α-Synuclein in Parkinson's Disease: From a Neuron-Glia Crosstalk Perspective.Int J Mol Sci. 2022 Nov 25;23(23):14753. doi: 10.3390/ijms232314753. Int J Mol Sci. 2022. PMID: 36499080 Free PMC article. Review.
-
Axonal Protection by Netarsudil, a ROCK Inhibitor, Is Linked to an AMPK-Autophagy Pathway in TNF-Induced Optic Nerve Degeneration.Invest Ophthalmol Vis Sci. 2022 Jan 3;63(1):4. doi: 10.1167/iovs.63.1.4. Invest Ophthalmol Vis Sci. 2022. PMID: 34982146 Free PMC article.
-
Parkinson's disease risk genes act in glia to control neuronal α-synuclein toxicity.Neurobiol Dis. 2021 Nov;159:105482. doi: 10.1016/j.nbd.2021.105482. Epub 2021 Aug 11. Neurobiol Dis. 2021. PMID: 34390834 Free PMC article.
-
Pathogenic Mutations Differentially Regulate Cell-to-Cell Transmission of α-Synuclein.Front Cell Neurosci. 2020 Jun 12;14:159. doi: 10.3389/fncel.2020.00159. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32595456 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

