Gab3 is required for IL-2- and IL-15-induced NK cell expansion and limits trophoblast invasion during pregnancy
- PMID: 31375526
- PMCID: PMC7068803
- DOI: 10.1126/sciimmunol.aav3866
Gab3 is required for IL-2- and IL-15-induced NK cell expansion and limits trophoblast invasion during pregnancy
Abstract
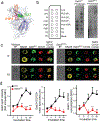
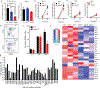
The scaffolding protein Grb2-associated binding protein 3 (Gab3) is a member of the Gab family, whose functions have remained elusive. Here, we identify Gab3 as a key determinant of peripheral NK cell expansion. Loss of Gab3 resulted in impaired IL-2 and IL-15-induced NK cell priming and expansion due to a selective impairment in MAPK signaling but not STAT5 signaling. In vivo, we found that Gab3 is required for recognition and elimination of "missing-self" and tumor targets. Unexpectedly, our studies also revealed that Gab3 plays an important role during pregnancy. Gab3-deficient mice exhibited impaired uterine NK cell expansion associated with abnormal spiral artery remodeling and increased trophoblast invasion in the decidua basalis. This coincided with stillbirth, retained placenta, maternal hemorrhage, and undelivered fetoplacental units at term. Thus, Gab3 is a key component required for cytokine-mediated NK cell priming and expansion that is essential for antitumor responses and limits trophoblast cell invasion during pregnancy.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

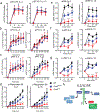
 ), Gab3KO (
), Gab3KO ( ) and NK cell-depleted (
) and NK cell-depleted ( ) mice were injected with 1×105 B16-F10 melanoma cells i.v.. After three weeks, mice were sacrificed, and tumor burden was determined (bars represent mean ± s.e.m). f) Frequency of dystocia observed in WT, Gab3R27C and Gab3KO mice. g,h) Representative images of stillborn pups, retained placentas (g) and maternal hemorrhaging (h) of a Gab3KO female with dystocia. Statistical analysis was performed using one-way ANOVA with Tukey post-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
) mice were injected with 1×105 B16-F10 melanoma cells i.v.. After three weeks, mice were sacrificed, and tumor burden was determined (bars represent mean ± s.e.m). f) Frequency of dystocia observed in WT, Gab3R27C and Gab3KO mice. g,h) Representative images of stillborn pups, retained placentas (g) and maternal hemorrhaging (h) of a Gab3KO female with dystocia. Statistical analysis was performed using one-way ANOVA with Tukey post-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001

Comment in
-
Placentation and antitumor immunity regulated by a scaffolding protein in NK cells.Sci Immunol. 2019 Aug 2;4(38):eaax9589. doi: 10.1126/sciimmunol.aax9589. Sci Immunol. 2019. PMID: 31375527 Review.
Similar articles
-
Placentation and antitumor immunity regulated by a scaffolding protein in NK cells.Sci Immunol. 2019 Aug 2;4(38):eaax9589. doi: 10.1126/sciimmunol.aax9589. Sci Immunol. 2019. PMID: 31375527 Review.
-
Natural killer-cell deficiency alters placental development in rats.Biol Reprod. 2017 Jan 1;96(1):145-158. doi: 10.1095/biolreprod.116.142752. Biol Reprod. 2017. PMID: 28395334 Free PMC article.
-
Trophoblasts regulate natural killer cells via control of interleukin-15 receptor signaling.Am J Reprod Immunol. 2017 Aug;78(2). doi: 10.1111/aji.12628. Epub 2017 Mar 2. Am J Reprod Immunol. 2017. PMID: 28251711
-
PGE2-mediated immunosuppression by first trimester human decidual cells blocks activation of maternal leukocytes in the decidua with potential anti-trophoblast activity.Cell Immunol. 1989 Apr 15;120(1):61-74. doi: 10.1016/0008-8749(89)90174-3. Cell Immunol. 1989. PMID: 2784722
-
Recognition of trophoblast HLA class I molecules by decidual NK cell receptors--a review.Placenta. 2000 Mar-Apr;21 Suppl A:S81-5. doi: 10.1053/plac.1999.0520. Placenta. 2000. PMID: 10831129 Review.
Cited by
-
Single-Cell RNA-Sequencing Reveals Interactions between Endometrial Stromal Cells, Epithelial Cells, and Lymphocytes during Mouse Embryo Implantation.Int J Mol Sci. 2022 Dec 22;24(1):213. doi: 10.3390/ijms24010213. Int J Mol Sci. 2022. PMID: 36613656 Free PMC article.
-
Comprehensive analysis of single cell and bulk RNA sequencing reveals the heterogeneity of melanoma tumor microenvironment and predicts the response of immunotherapy.Inflamm Res. 2024 Aug;73(8):1393-1409. doi: 10.1007/s00011-024-01905-5. Epub 2024 Jun 19. Inflamm Res. 2024. PMID: 38896289
-
NK cells for cancer immunotherapy.Nat Rev Drug Discov. 2020 Mar;19(3):200-218. doi: 10.1038/s41573-019-0052-1. Epub 2020 Jan 6. Nat Rev Drug Discov. 2020. PMID: 31907401 Review.
-
Animal models of the placenta accreta spectrum: current status and further perspectives.Front Endocrinol (Lausanne). 2023 May 8;14:1118168. doi: 10.3389/fendo.2023.1118168. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37223034 Free PMC article. Review.
-
Candidate genes for infertility: an in-silico study based on cytogenetic analysis.BMC Med Genomics. 2022 Aug 2;15(1):170. doi: 10.1186/s12920-022-01320-x. BMC Med Genomics. 2022. PMID: 35918717 Free PMC article.
References
-
- Lemmon MA, Ferguson KM, Molecular determinants in pleckstrin homology domains that allow specific recognition of phosphoinositides. Biochem Soc Trans 29, 377–384 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

