Topical Inserts: A Versatile Delivery Form for HIV Prevention
- PMID: 31374941
- PMCID: PMC6723036
- DOI: 10.3390/pharmaceutics11080374
Topical Inserts: A Versatile Delivery Form for HIV Prevention
Abstract
The development of topical inserts for the prevention of sexually transmitted infections (STIs), particularly human immunodeficiency virus (HIV), represents a promising alternative to oral and parenteral pre-exposure prophylaxis (PrEP) dosage forms. They may be used for vaginal and/or rectal administration of a variety of agents with antiviral activity. Topical inserts deliver drugs to the portal of viral entry, i.e., the genital or rectal mucosa, with low systemic exposure, and therefore are safer and have fewer side effects than systemic PrEP agents. They may dissolve fast, releasing the active drugs within minutes of insertion, or slowly for long-acting drug delivery. Furthermore, they are user-friendly being easy to administer, discreet and highly portable. They are also economical and easy to manufacture at scale and to distribute, with excellent stability and shelf-life. Altogether, topical inserts represent a particularly promising form of drug delivery for HIV and STI prevention. Highlighted within this review are end-user acceptability research dedicated to understanding preferred attributes for this form of drug delivery, advantages and disadvantages of the formulation platform options, considerations for their development, clinical assessment of select placebo prototypes, future directions, and the potential impact of this dosage form on the HIV prevention landscape.
Keywords: HIV; extended-release; fast-disintegrating; microbicides; on-demand; protection; rectal drug delivery; tablets; vaginal drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
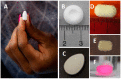
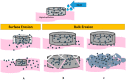


Similar articles
-
Preclinical and Early Clinical Development of Tenofovir Alafenamide/Elvitegravir Topical Inserts for Effective On-Demand Vaginal and Rectal HIV Prevention.Pharmaceutics. 2024 Mar 1;16(3):348. doi: 10.3390/pharmaceutics16030348. Pharmaceutics. 2024. PMID: 38543242 Free PMC article.
-
Clinical development of microbicides for the prevention of HIV infection.Curr Pharm Des. 2004;10(3):315-36. doi: 10.2174/1381612043386374. Curr Pharm Des. 2004. PMID: 14754390 Review.
-
Perceptions among Dutch men who have sex with men and their willingness to use rectal microbicides and oral pre-exposure prophylaxis to reduce HIV risk--a preliminary study.AIDS Care. 2015;27(12):1493-500. doi: 10.1080/09540121.2015.1069785. AIDS Care. 2015. PMID: 26695133
-
Modeling HIV Pre-Exposure Prophylaxis.Front Pharmacol. 2020 Jan 31;10:1514. doi: 10.3389/fphar.2019.01514. eCollection 2019. Front Pharmacol. 2020. PMID: 32082142 Free PMC article. Review.
-
Acceptability and performance of a nonwoven device for vaginal drug delivery among women and their male partners in KwaZulu-Natal, South Africa.Eur J Contracept Reprod Health Care. 2019 Oct;24(5):390-398. doi: 10.1080/13625187.2019.1656188. Epub 2019 Sep 13. Eur J Contracept Reprod Health Care. 2019. PMID: 31517545
Cited by
-
Women for science and science for women: Gaps, challenges and opportunities towards optimizing pre-exposure prophylaxis for HIV-1 prevention.Front Immunol. 2022 Dec 6;13:1055042. doi: 10.3389/fimmu.2022.1055042. eCollection 2022. Front Immunol. 2022. PMID: 36561760 Free PMC article. Review.
-
A phase I study to assess safety, pharmacokinetics, and pharmacodynamics of a vaginal insert containing tenofovir alafenamide and elvitegravir.Front Cell Infect Microbiol. 2023 Apr 19;13:1130101. doi: 10.3389/fcimb.2023.1130101. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37153145 Free PMC article. Clinical Trial.
-
Co-crystals, Salts or Mixtures of Both? The Case of Tenofovir Alafenamide Fumarates.Pharmaceutics. 2020 Apr 10;12(4):342. doi: 10.3390/pharmaceutics12040342. Pharmaceutics. 2020. PMID: 32290280 Free PMC article.
-
A Phase 1 Clinical Trial to Assess the Safety and Pharmacokinetics of a Tenofovir Alafenamide/Elvitegravir Insert Administered Rectally for HIV Prevention.J Infect Dis. 2024 Sep 23;230(3):696-705. doi: 10.1093/infdis/jiae211. J Infect Dis. 2024. PMID: 38655842 Clinical Trial.
-
Extended Postexposure Protection Against Vaginal Simian/Human Immunodeficiency Virus Infection With Tenofovir Alafenamide Fumarate/Elvitegravir Inserts in Macaques.J Infect Dis. 2024 Jun 14;229(6):1791-1795. doi: 10.1093/infdis/jiad599. J Infect Dis. 2024. PMID: 38134382 Free PMC article.
References
-
- Joint United Nations Programme on HIV/AIDS Fact Sheet—Latest Statistics on the Status of the AIDS Epidemic. [(accessed on 24 July 2019)]; Available online: http://www.unaids.org/en/resources/fact-sheet.
-
- UNAIDS . Ambitious Treatment Targets: Writing the Final Chapter of the AIDS Epidemic. UNAIDS; Geneva, Switzerland: 2014.
-
- UNAIDS. Sabin K. The Prevention Gap Report. UNAIDS; Geneva, Switzerland: 2016.
-
- Grant R.M., Lama J.R., Anderson P.L., McMahan V., Liu A.Y., Vargas L., Goicochea P., Casapia M., Guanira-Carranza J.V., Ramirez-Cardich M.E., et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

