Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial
- PMID: 31373537
- PMCID: PMC7158919
- DOI: 10.1080/21645515.2019.1651141
Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial
Abstract
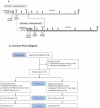
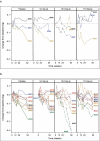
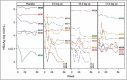
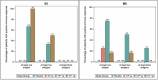
Treatment of chronic hepatitis B (CHB) typically requires life-long administration of drugs. Cohort and pre-clinical studies have established the link between a functional T-cell-mounted immunity and resolution of infection. TG1050 is an adenovirus 5-based vaccine that expresses HBV polymerase and domains of core and surface antigen and has shown immunogenicity and antiviral effects in mice. We performed a phase 1 clinical trial to assess safety and explore immunogenicity and early efficacy of TG1050 in CHB patients. This randomized, double blind, placebo-controlled study included two sequential phases: one single dose cohort (SD, n = 12) and one multiple (3) doses cohort (MD, n = 36). Patients, virally suppressed under nucleoside(d)tide analog NUC therapy, were randomized 1:1:1 across 3 dose levels (DL) and assigned to receive 109, 1010, 1011 virus particles (vp) of TG1050 and then randomized within each DL to placebo (3:1 and 9:3 vaccines/placebo in each DL, respectively, for the SD and MD cohorts). Cellular (ELISPOT) and antibody responses (anti-Adenovirus), as well as evolution of circulating HBsAg and HBcrAg, were monitored. All doses were well tolerated in both cohorts, without severe adverse event. TG1050 was capable to induce IFN-γ producing T-cells targeting 1 to 3 encoded antigens, in particular at the 1010vp dose. Overall, minor decreases of HBsAg were observed while a number of vaccinees reached unquantifiable HBcrAg by end of the study. In CHB patients under NUC, TG1050 exhibited a good safety profile and was capable to induce HBV-specific cellular immune response. These data support further clinical evaluation, especially in combination studies.
Keywords: Hepatitis B; chronicity; immuno-therapy; immunogenicity; safety; vaccine.
Figures

Similar articles
-
Recognition of Core- and Polymerase-derived immunogenic peptides included in novel therapeutic vaccine by T cells from Chinese chronic hepatitis B patients.J Viral Hepat. 2017 Nov;24 Suppl 1:66-74. doi: 10.1111/jvh.12791. J Viral Hepat. 2017. PMID: 29082648
-
TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T cells and exerts an antiviral effect in HBV-persistent mice.Gut. 2015 Dec;64(12):1961-71. doi: 10.1136/gutjnl-2014-308041. Epub 2014 Nov 26. Gut. 2015. PMID: 25429051 Free PMC article.
-
Therapeutic vaccine BRII-179 restores HBV-specific immune responses in patients with chronic HBV in a phase Ib/IIa study.JHEP Rep. 2021 Sep 8;3(6):100361. doi: 10.1016/j.jhepr.2021.100361. eCollection 2021 Dec. JHEP Rep. 2021. PMID: 34661089 Free PMC article.
-
HBsAg, HBcAg, and combined HBsAg/HBcAg-based therapeutic vaccines in treating chronic hepatitis B virus infection.Hepatobiliary Pancreat Dis Int. 2013 Aug;12(4):363-9. doi: 10.1016/s1499-3872(13)60057-0. Hepatobiliary Pancreat Dis Int. 2013. PMID: 23924493 Review.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047 Review.
Cited by
-
Designing the next-generation therapeutic vaccines to cure chronic hepatitis B: focus on antigen presentation, vaccine properties and effect measures.Clin Transl Immunology. 2021 Jan 15;10(1):e1232. doi: 10.1002/cti2.1232. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33489122 Free PMC article. Review.
-
Analysis of the Prevalence of Binding and Neutralizing Antibodies against 39 Human Adenovirus Types in Student Cohorts Reveals Low-Prevalence Types and a Decline in Binding Antibody Levels during the SARS-CoV-2 Pandemic.J Virol. 2022 Nov 23;96(22):e0113322. doi: 10.1128/jvi.01133-22. Epub 2022 Nov 7. J Virol. 2022. PMID: 36342295 Free PMC article.
-
Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure.Gastroenterology. 2023 Jan;164(1):42-60.e6. doi: 10.1053/j.gastro.2022.10.008. Epub 2022 Oct 12. Gastroenterology. 2023. PMID: 36243037 Free PMC article. Review.
-
[Vaccination against hepatitis B as prevention for hepatocellular carcinoma].Onkologe (Berl). 2022;28(1):15-22. doi: 10.1007/s00761-021-01036-0. Epub 2021 Oct 13. Onkologe (Berl). 2022. PMID: 34658542 Free PMC article. Review. German.
-
New Approaches to the Treatment of Chronic Hepatitis B.J Clin Med. 2020 Oct 1;9(10):3187. doi: 10.3390/jcm9103187. J Clin Med. 2020. PMID: 33019573 Free PMC article. Review.
References
-
- World Health Organization . 2017. [accessed 2019 July 19]. http://www.who.int/mediacentre/factsheets/fs204/en/
-
- Maini MK, Boni C, Ogg GS, King AS, Reignat AS, Lee CK, Larrubia JR, Webster GJ, McMichael AJ, Ferrari C, et al. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117:1386–96. doi:10.1016/s0016-5085(99)70289-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
