Role of AIM2 inflammasome in inflammatory diseases, cancer and infection
- PMID: 31372985
- PMCID: PMC7015662
- DOI: 10.1002/eji.201848070
Role of AIM2 inflammasome in inflammatory diseases, cancer and infection
Abstract
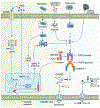
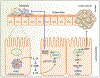
AIM2 is a cytosolic innate immune receptor which recognizes double-stranded DNA (dsDNA) released during cellular perturbation and pathogenic assault. AIM2 recognition of dsDNA leads to the assembly of a large multiprotein oligomeric complex termed the inflammasome. This inflammasome assembly leads to the secretion of bioactive interleukin-1β (IL-1β) and IL-18 and induction of an inflammatory form of cell death called pyroptosis. Sensing of dsDNA by AIM2 in the cytosol is crucial to mediate protection against the invading pathogens including bacteria, virus, fungi and parasites. AIM2 also responds to dsDNA released from damaged host cells, resulting in the secretion of the effector cytokines thereby driving the progression of sterile inflammatory diseases such as skin disease, neuronal disease, chronic kidney disease, cardiovascular disease and diabetes. Additionally, the protection mediated by AIM2 in the development of colorectal cancer depends on its ability to regulate epithelial cell proliferation and gut microbiota in maintaining intestinal homeostasis independently of the effector cytokines. In this review, we will highlight the recent progress on the role of the AIM2 inflammasome as a guardian of cellular integrity in modulating chronic inflammatory diseases, cancer and infection.
Keywords: AIM2; cell death; gasdermin; inflammasome; pyroptosis.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflicts of Interest
The authors declare no commercial or financial conflict of interest to disclose.
Figures
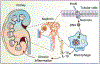

Similar articles
-
The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection.Biochim Biophys Acta Rev Cancer. 2019 Aug;1872(1):1-10. doi: 10.1016/j.bbcan.2019.05.001. Epub 2019 May 3. Biochim Biophys Acta Rev Cancer. 2019. PMID: 31059737 Review.
-
Cytokine secretion and pyroptosis of cholesteatoma keratinocytes mediated by AIM2 inflammasomes in response to cytoplasmic DNA.Mol Med Rep. 2021 May;23(5):344. doi: 10.3892/mmr.2021.11983. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760111 Free PMC article.
-
The AIM2 inflammasome: Sensor of pathogens and cellular perturbations.Immunol Rev. 2018 Jan;281(1):99-114. doi: 10.1111/imr.12618. Immunol Rev. 2018. PMID: 29247998 Review.
-
Identification of Aim2 as a sensor for DNA vaccines.J Immunol. 2015 Jan 15;194(2):630-6. doi: 10.4049/jimmunol.1402530. Epub 2014 Dec 8. J Immunol. 2015. PMID: 25488991 Free PMC article.
-
AIM2 senses Brucella abortus DNA in dendritic cells to induce IL-1β secretion, pyroptosis and resistance to bacterial infection in mice.Microbes Infect. 2019 Mar;21(2):85-93. doi: 10.1016/j.micinf.2018.09.001. Epub 2018 Sep 22. Microbes Infect. 2019. PMID: 30248400 Free PMC article.
Cited by
-
Overexpression of NAG-1/GDF15 prevents hepatic steatosis through inhibiting oxidative stress-mediated dsDNA release and AIM2 inflammasome activation.Redox Biol. 2022 Jun;52:102322. doi: 10.1016/j.redox.2022.102322. Epub 2022 Apr 27. Redox Biol. 2022. PMID: 35504134 Free PMC article.
-
The P2X7R-NLRP3 and AIM2 Inflammasome Platforms Mark the Complexity/Severity of Viral or Metabolic Liver Damage.Int J Mol Sci. 2022 Jul 4;23(13):7447. doi: 10.3390/ijms23137447. Int J Mol Sci. 2022. PMID: 35806450 Free PMC article.
-
PYHIN1 regulates pro-inflammatory cytokine induction rather than innate immune DNA sensing in airway epithelial cells.J Biol Chem. 2020 Apr 3;295(14):4438-4450. doi: 10.1074/jbc.RA119.011400. Epub 2020 Feb 26. J Biol Chem. 2020. PMID: 32102850 Free PMC article.
-
Haemolysins are essential to the pathogenicity of deep-sea Vibrio fluvialis.iScience. 2024 Mar 26;27(5):109558. doi: 10.1016/j.isci.2024.109558. eCollection 2024 May 17. iScience. 2024. PMID: 38650982 Free PMC article.
-
Role of the inflammasome in insulin resistance and type 2 diabetes mellitus.Front Immunol. 2023 Mar 13;14:1052756. doi: 10.3389/fimmu.2023.1052756. eCollection 2023. Front Immunol. 2023. PMID: 36993972 Free PMC article. Review.
References
-
- Takeuchi O and Akira S, Pattern recognition receptors and inflammation. Cell 2010. 140: 805–820. - PubMed
-
- Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL and Superti-Furga G, An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 2009. 10: 266–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

