Real-life management of patients with breakthrough cancer pain caused by bone metastases in Spain
- PMID: 31372030
- PMCID: PMC6636433
- DOI: 10.2147/JPR.S194881
Real-life management of patients with breakthrough cancer pain caused by bone metastases in Spain
Abstract
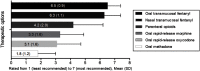
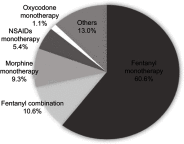
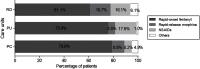
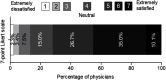
Purpose: We aimed to explore the characteristics, and real-life therapeutic management of patients with breakthrough cancer pain (BTcP) caused by bone metastases in Spain, and to evaluate physicians' opinion of and satisfaction with prescribed BTcP therapy. Participants and methods: For the purposes of this study, an ad-hoc questionnaire was developed consisting of two domains: a) organizational aspects and care standards; b) clinical and treatment variables of bone metastatic BTcP patients. In addition, physicians' satisfaction with their prescribed BTcP therapy was assessed. Specialists collected data from up to five patients receiving treatment for BTcP caused by bone metastasis, all patients gave their consent to participate prior to inclusion. Results: A total of 103 cancer pain specialists (radiation oncologists [38.8%], pain specialists [33.0%], and palliative care (PC) specialists [21.4%]) were polled, and data on 386 BTcP patients with bone metastatic disease were collected. Only 33% of the specialists had implemented specific protocols for BTcP management, and 19.4% had established referral protocols for this group of patients. Half of all participants (50.5%) address quality of life and quality of care in their patients; however, only 27.0% did so from the patient's perspective, as they should do. Most patients had multiple metastases and were prescribed rapid-onset fentanyl preparations (71.2%), followed by immediate-release morphine (9.3%) for the treatment of BTcP. Rapid-onset fentanyl was prescribed more often in PC units (79.0%) than in pain units (75.9%) and radiation oncology units (61.1%) (p<0.01). Furthermore, most physicians (71.8%) were satisfied with the BTcP therapy prescribed. Conclusions: Our results demonstrate the need for routine assessment of quality of life in patients with bone BTcP. These findings also underscore the necessity for a multidisciplinary therapeutic strategy for breakthrough pain in clinical practice in Spain.
Keywords: bone metastases; breakthrough cancer pain; health-related quality of life; management; opioids; satisfaction.
Conflict of interest statement
CFA and FVE received fees from Kyowa Kirin Farmacéutica, S.L.U., for the design, execution, and coordination of the study. In addition, the following authors declare potential conflicts of interest for individual activities for the industry outside the submitted work: CFA has received payments for consultancies and lecture fees from Astellas Pharma, Janssen, GP-Pharm, and Kyowa Kirin Farmacéutica, S.L.U. FVE has received payments for consultancies and lecture fees from Grünenthal, Gebro Pharma, Menarini, Takeda, Mylan, Esteve, and Ferrer. MDLA has received payments for consultancies and lecture fees from Kyowa Kirin Farmacéutica, S.L.U., Takeda, Gebro Pharma, Esteve, Teva, and Grünenthal. MM has received lecture fees from Kyowa Kirin Farmacéutica, S.L.U., Grünenthal, Mundipharma, Ferrer, and Medtronic. AC has received payments for consultancies and lecture fees from Takeda, Gebro Pharma, Esteve, Teva, Grünenthal, and Kyowa Kirin Farmacéutica, S.L.U. JA has received payments for consultancies and lecture fees from Esteve, Abstral and Kyowa Kirin Farmacéutica, S.L.U. JMTMA has received payments for consultancies and lecture fees from Grünenthal, Boston Scientific, Gebro Pharma, Angellini Farmaceutica, Takeda, Kyowa Kirin Farmacéutica, S.L.U. and Ferrer. AJJL and ASY are employed by Kyowa Kirin Farmacéutica, S.L.U. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Current management of breakthrough cancer pain according to physicians from pain units in Spain.Clin Transl Oncol. 2019 Sep;21(9):1168-1176. doi: 10.1007/s12094-019-02044-8. Epub 2019 Feb 19. Clin Transl Oncol. 2019. PMID: 30783918
-
Breakthrough cancer pain treatment in Spain: physicians' perception of current opioids utilization and prescription.Curr Med Res Opin. 2020 Aug;36(8):1383-1391. doi: 10.1080/03007995.2020.1775073. Epub 2020 Jun 11. Curr Med Res Opin. 2020. PMID: 32453602
-
Breakthrough cancer pain tailored treatment: which factors influence the medication choice? An observational, prospective and cross-sectional study in patients with terminal cancer.Postgrad Med J. 2018 Oct;94(1116):566-570. doi: 10.1136/postgradmedj-2018-135659. Epub 2018 Oct 13. Postgrad Med J. 2018. PMID: 30317182
-
Expert consensus on the management of breakthrough cancer pain in older patients. A Delphi study.J Geriatr Oncol. 2019 Jul;10(4):643-652. doi: 10.1016/j.jgo.2019.03.012. Epub 2019 Apr 26. J Geriatr Oncol. 2019. PMID: 31036463
-
Efficacy of rapid-onset oral fentanyl formulations vs. oral morphine for cancer-related breakthrough pain: a meta-analysis of comparative trials.J Pain Symptom Manage. 2013 Oct;46(4):573-80. doi: 10.1016/j.jpainsymman.2012.09.009. Epub 2013 Feb 4. J Pain Symptom Manage. 2013. PMID: 23380337 Review.
Cited by
-
Carbon fibers for treatment of cancer metastasis in bone.RSC Adv. 2020 Sep 7;10(55):33071-33079. doi: 10.1039/d0ra05992g. eCollection 2020 Sep 7. RSC Adv. 2020. PMID: 35515018 Free PMC article.
-
High-rate breakthrough cancer pain and tumour characteristics - literature review and case series.Drugs Context. 2023 Mar 10;12:2022-11-1. doi: 10.7573/dic.2022-11-1. eCollection 2023. Drugs Context. 2023. PMID: 36926050 Free PMC article. Review.
-
Bayesian Network Analysis for Prediction of Unplanned Hospital Readmissions of Cancer Patients with Breakthrough Cancer Pain and Complex Care Needs.Healthcare (Basel). 2022 Sep 23;10(10):1853. doi: 10.3390/healthcare10101853. Healthcare (Basel). 2022. PMID: 36292299 Free PMC article.
-
Fentanyl in cancer pain management: avoiding hasty judgments and discerning its potential benefits.Drugs Context. 2023 Dec 14;12:2023-10-2. doi: 10.7573/dic.2023-10-2. eCollection 2023. Drugs Context. 2023. PMID: 38148830 Free PMC article. Review.
References
-
- Reyes Chiquete D ,Ortiz C González, Betancourt A Mohar, García A Meneses. Epidemiología del dolor por cáncer. Revista De La Sociedad Española Del Dolor. 2011;18:118–134.
LinkOut - more resources
Full Text Sources

