The Hajdu Cheney mutation sensitizes mice to the osteolytic actions of tumor necrosis factor α
- PMID: 31371452
- PMCID: PMC6768656
- DOI: 10.1074/jbc.RA119.009824
The Hajdu Cheney mutation sensitizes mice to the osteolytic actions of tumor necrosis factor α
Abstract
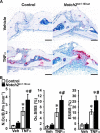
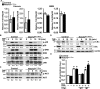
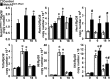
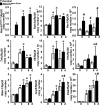
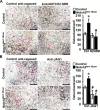
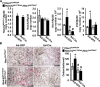
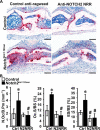
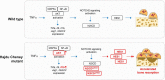
Hajdu Cheney syndrome (HCS) is characterized by craniofacial developmental abnormalities, acro-osteolysis, and osteoporosis and is associated with gain-of-NOTCH2 function mutations. A mouse model of HCS termed Notch2tm1.1Ecan harboring a mutation in exon 34 of Notch2 replicating the one found in HCS was used to determine whether the HCS mutation sensitizes the skeleton to the osteolytic effects of tumor necrosis factor α (TNFα). TNFα injected over the calvarial vault caused a greater increase in osteoclast number, osteoclast surface, and eroded surface in Notch2tm1.1Ecan mice compared with littermate WT controls. Accordingly, the effect of TNFα on osteoclastogenesis was greatly enhanced in cultures of bone marrow-derived macrophages (BMMs) from Notch2tm1.1Ecan mice when compared with the activity of TNFα in control cultures. TNFα induced the expression of Notch2 and Notch2 mutant mRNA by ∼2-fold, possibly amplifying the NOTCH2-dependent induction of osteoclastogenesis. The effect of TNFα on osteoclastogenesis in Notch2tm1.1Ecan mutants depended on NOTCH2 activation because it was reversed by anti-NOTCH2 negative regulatory region and anti-jagged 1 antibodies. The inactivation of Hes1 prevented the TNFα effect on osteoclastogenesis in the context of the Notch2tm1.1Ecan mutation. In addition, the induction of Il1b, but not of Tnfa and Il6, mRNA by TNFα was greater in Notch2tm1.1Ecan BMMs than in control cells, possibly contributing to the actions of TNFα and NOTCH2 on osteoclastogenesis. In conclusion, the HCS mutation enhances TNFα-induced osteoclastogenesis and the inflammatory bone-resorptive response possibly explaining the acro-osteolysis observed in affected individuals.
Keywords: Hajdu Cheney syndrome; Notch receptor; cytokine; inflammation; osteoclast; osteolysis; tumor necrosis factor (TNF).
© 2019 Yu and Canalis.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Antisense oligonucleotides targeting Notch2 ameliorate the osteopenic phenotype in a mouse model of Hajdu-Cheney syndrome.J Biol Chem. 2020 Mar 20;295(12):3952-3964. doi: 10.1074/jbc.RA119.011440. Epub 2020 Jan 28. J Biol Chem. 2020. PMID: 31992595 Free PMC article.
-
Hairy and enhancer of split 1 is a primary effector of NOTCH2 signaling and induces osteoclast differentiation and function.J Biol Chem. 2021 Dec;297(6):101376. doi: 10.1016/j.jbc.2021.101376. Epub 2021 Nov 3. J Biol Chem. 2021. PMID: 34742737 Free PMC article.
-
Mice harboring a Hajdu Cheney Syndrome mutation are sensitized to osteoarthritis.Bone. 2018 Sep;114:198-205. doi: 10.1016/j.bone.2018.06.020. Epub 2018 Jun 22. Bone. 2018. PMID: 29940267 Free PMC article.
-
Hajdu-Cheney Syndrome, a Disease Associated with NOTCH2 Mutations.Curr Osteoporos Rep. 2016 Aug;14(4):126-31. doi: 10.1007/s11914-016-0311-6. Curr Osteoporos Rep. 2016. PMID: 27241678 Free PMC article. Review.
-
Hajdu-Cheney syndrome: a review.Orphanet J Rare Dis. 2014 Dec 10;9:200. doi: 10.1186/s13023-014-0200-y. Orphanet J Rare Dis. 2014. PMID: 25491639 Free PMC article. Review.
Cited by
-
Progress and Current Status in Hajdu-Cheney Syndrome with Focus on Novel Genetic Research.Int J Mol Sci. 2022 Sep 27;23(19):11374. doi: 10.3390/ijms231911374. Int J Mol Sci. 2022. PMID: 36232677 Free PMC article. Review.
-
Fracture healing in a mouse model of Hajdu-Cheney-Syndrome with high turnover osteopenia results in decreased biomechanical stability.Sci Rep. 2023 Jul 14;13(1):11418. doi: 10.1038/s41598-023-38638-0. Sci Rep. 2023. PMID: 37452111 Free PMC article.
-
The Skeleton of Lateral Meningocele Syndrome.Front Genet. 2021 Jan 14;11:620334. doi: 10.3389/fgene.2020.620334. eCollection 2020. Front Genet. 2021. PMID: 33519922 Free PMC article. Review.
-
Distinct severity of phenotype in Hajdu-Cheney syndrome: a case report and literature review.BMC Musculoskelet Disord. 2020 Mar 6;21(1):154. doi: 10.1186/s12891-020-3181-0. BMC Musculoskelet Disord. 2020. PMID: 32143606 Free PMC article. Review.
-
Antisense oligonucleotides targeting Notch2 ameliorate the osteopenic phenotype in a mouse model of Hajdu-Cheney syndrome.J Biol Chem. 2020 Mar 20;295(12):3952-3964. doi: 10.1074/jbc.RA119.011440. Epub 2020 Jan 28. J Biol Chem. 2020. PMID: 31992595 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

