Increased autophagy in EphrinB2-deficient osteocytes is associated with elevated secondary mineralization and brittle bone
- PMID: 31366886
- PMCID: PMC6668467
- DOI: 10.1038/s41467-019-11373-9
Increased autophagy in EphrinB2-deficient osteocytes is associated with elevated secondary mineralization and brittle bone
Erratum in
-
Author Correction: Increased autophagy in EphrinB2-deficient osteocytes is associated with elevated secondary mineralization and brittle bone.Nat Commun. 2019 Nov 4;10(1):5073. doi: 10.1038/s41467-019-13040-5. Nat Commun. 2019. PMID: 31685818 Free PMC article.
Abstract
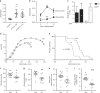
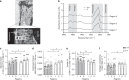
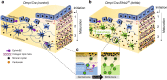
Mineralized bone forms when collagen-containing osteoid accrues mineral crystals. This is initiated rapidly (primary mineralization), and continues slowly (secondary mineralization) until bone is remodeled. The interconnected osteocyte network within the bone matrix differentiates from bone-forming osteoblasts; although osteoblast differentiation requires EphrinB2, osteocytes retain its expression. Here we report brittle bones in mice with osteocyte-targeted EphrinB2 deletion. This is not caused by low bone mass, but by defective bone material. While osteoid mineralization is initiated at normal rate, mineral accrual is accelerated, indicating that EphrinB2 in osteocytes limits mineral accumulation. No known regulators of mineralization are modified in the brittle cortical bone but a cluster of autophagy-associated genes are dysregulated. EphrinB2-deficient osteocytes displayed more autophagosomes in vivo and in vitro, and EphrinB2-Fc treatment suppresses autophagy in a RhoA-ROCK dependent manner. We conclude that secondary mineralization involves EphrinB2-RhoA-limited autophagy in osteocytes, and disruption leads to a bone fragility independent of bone mass.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Cellular Processes by Which Osteoblasts and Osteocytes Control Bone Mineral Deposition and Maturation Revealed by Stage-Specific EphrinB2 Knockdown.Curr Osteoporos Rep. 2019 Oct;17(5):270-280. doi: 10.1007/s11914-019-00524-y. Curr Osteoporos Rep. 2019. PMID: 31401710 Review.
-
EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone.J Bone Miner Res. 2013 Apr;28(4):912-25. doi: 10.1002/jbmr.1820. J Bone Miner Res. 2013. PMID: 23165727
-
Mechanism by which MLO-A5 late osteoblasts/early osteocytes mineralize in culture: similarities with mineralization of lamellar bone.Calcif Tissue Int. 2006 Nov;79(5):340-53. doi: 10.1007/s00223-006-0107-2. Epub 2006 Nov 14. Calcif Tissue Int. 2006. PMID: 17115241 Free PMC article.
-
Functions of Rho family of small GTPases and Rho-associated coiled-coil kinases in bone cells during differentiation and mineralization.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt A):1009-1023. doi: 10.1016/j.bbagen.2017.02.005. Epub 2017 Feb 8. Biochim Biophys Acta Gen Subj. 2017. PMID: 28188861 Review.
-
Osteocytes but not osteoblasts directly build mineralized bone structures.Int J Biol Sci. 2021 Jun 11;17(10):2430-2448. doi: 10.7150/ijbs.61012. eCollection 2021. Int J Biol Sci. 2021. PMID: 34326685 Free PMC article.
Cited by
-
EphrinB2-mediated chondrocyte autophagy induces post-traumatic arthritis via rupture of cartilage homeostasis.J Cell Mol Med. 2024 Sep;28(18):e70095. doi: 10.1111/jcmm.70095. J Cell Mol Med. 2024. PMID: 39289794 Free PMC article.
-
Osteoclast-derived coupling factors: origins and state-of-play Louis V Avioli lecture, ASBMR 2023.J Bone Miner Res. 2024 Sep 26;39(10):1377-1385. doi: 10.1093/jbmr/zjae110. J Bone Miner Res. 2024. PMID: 38990205 Free PMC article. Review.
-
Exploring the Osteogenic Potential of Zinc-Doped Magnesium Phosphate Cement (ZMPC): A Novel Material for Orthopedic Bone Defect Repair.Biomedicines. 2024 Feb 1;12(2):344. doi: 10.3390/biomedicines12020344. Biomedicines. 2024. PMID: 38397946 Free PMC article.
-
Genetic and Gene Expression Resources for Osteoporosis and Bone Biology Research.Curr Osteoporos Rep. 2023 Dec;21(6):637-649. doi: 10.1007/s11914-023-00821-7. Epub 2023 Oct 13. Curr Osteoporos Rep. 2023. PMID: 37831357 Free PMC article. Review.
-
Autophagy, a double-edged sword for oral tissue regeneration.J Adv Res. 2024 May;59:141-159. doi: 10.1016/j.jare.2023.06.010. Epub 2023 Jun 24. J Adv Res. 2024. PMID: 37356803 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

