The ventral epithelium of Trichoplax adhaerens deploys in distinct patterns cells that secrete digestive enzymes, mucus or diverse neuropeptides
- PMID: 31366453
- PMCID: PMC6737977
- DOI: 10.1242/bio.045674
The ventral epithelium of Trichoplax adhaerens deploys in distinct patterns cells that secrete digestive enzymes, mucus or diverse neuropeptides
Abstract
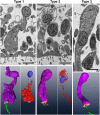
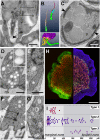
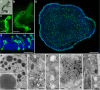
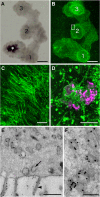
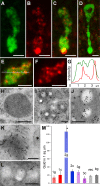
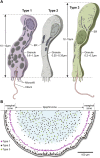
The disk-shaped millimeter-sized marine animal, Trichoplax adhaerens, is notable because of its small number of cell types and primitive mode of feeding. It glides on substrates propelled by beating cilia on its lower surface and periodically pauses to feed on underlying microorganisms, which it digests externally. Here, a combination of advanced electron and light microscopic techniques are used to take a closer look at its secretory cell types and their roles in locomotion and feeding. We identify digestive enzymes in lipophils, a cell type implicated in external digestion and distributed uniformly throughout the ventral epithelium except for a narrow zone near its edge. We find three morphologically distinct types of gland cell. The most prevalent contains and secretes mucus, which is shown to be involved in adhesion and gliding. Half of the mucocytes are arrayed in a tight row around the edge of the ventral epithelium while the rest are scattered further inside, in the region containing lipophils. The secretory granules in mucocytes at the edge label with an antibody against a neuropeptide that was reported to arrest ciliary beating during feeding. A second type of gland cell is arrayed in a narrow row just inside the row of mucocytes while a third is located more centrally. Our maps of the positions of the structurally distinct secretory cell types provide a foundation for further characterization of the multiple peptidergic cell types in Trichoplax and the microscopic techniques we introduce provide tools for carrying out these studies.
Keywords: Digestive system evolution; Gland cell; Mucus; Nervous system evolution; Neuropeptide; Placozoa.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
Neuropeptidergic integration of behavior in Trichoplax adhaerens, an animal without synapses.J Exp Biol. 2017 Sep 15;220(Pt 18):3381-3390. doi: 10.1242/jeb.162396. J Exp Biol. 2017. PMID: 28931721 Free PMC article.
-
Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens.Curr Biol. 2014 Jul 21;24(14):1565-1572. doi: 10.1016/j.cub.2014.05.046. Epub 2014 Jun 19. Curr Biol. 2014. PMID: 24954051 Free PMC article.
-
Coordinated Feeding Behavior in Trichoplax, an Animal without Synapses.PLoS One. 2015 Sep 2;10(9):e0136098. doi: 10.1371/journal.pone.0136098. eCollection 2015. PLoS One. 2015. PMID: 26333190 Free PMC article.
-
Insights into the evolution of digestive systems from studies of Trichoplax adhaerens.Cell Tissue Res. 2019 Sep;377(3):353-367. doi: 10.1007/s00441-019-03057-z. Epub 2019 Jul 3. Cell Tissue Res. 2019. PMID: 31270610 Review.
-
Placozoa and the evolution of Metazoa and intrasomatic cell differentiation.Int J Biochem Cell Biol. 2009 Feb;41(2):370-9. doi: 10.1016/j.biocel.2008.09.023. Epub 2008 Oct 2. Int J Biochem Cell Biol. 2009. PMID: 18935972 Review.
Cited by
-
Alternative neural systems: What is a neuron? (Ctenophores, sponges and placozoans).Front Cell Dev Biol. 2022 Dec 23;10:1071961. doi: 10.3389/fcell.2022.1071961. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36619868 Free PMC article.
-
Function and phylogeny support the independent evolution of an ASIC-like Deg/ENaC channel in the Placozoa.Commun Biol. 2023 Sep 18;6(1):951. doi: 10.1038/s42003-023-05312-0. Commun Biol. 2023. PMID: 37723223 Free PMC article.
-
Cellular adaptations leading to coral fragment attachment on artificial substrates in Acropora millepora (Am-CAM).Sci Rep. 2022 Nov 1;12(1):18431. doi: 10.1038/s41598-022-23134-8. Sci Rep. 2022. PMID: 36319668 Free PMC article.
-
From Species to Regional and Local Specialization of Intestinal Macrophages.Front Cell Dev Biol. 2021 Feb 18;8:624213. doi: 10.3389/fcell.2020.624213. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33681185 Free PMC article. Review.
-
Conserved biophysical features of the CaV2 presynaptic Ca2+ channel homologue from the early-diverging animal Trichoplax adhaerens.J Biol Chem. 2020 Dec 25;295(52):18553-18578. doi: 10.1074/jbc.RA120.015725. Epub 2020 Oct 23. J Biol Chem. 2020. PMID: 33097592 Free PMC article.
References
-
- Behrendt G. and Ruthmann A. (1986). The cytoskeleton of the fiber cells of Trichoplax adhaerens (Placozoa). Zoomorphology 106, 123-130. 10.1007/BF00312114 - DOI
LinkOut - more resources
Full Text Sources

