High sporulation and overexpression of virulence factors in biofilms and reduced susceptibility to vancomycin and linezolid in recurrent Clostridium [Clostridioides] difficile infection isolates
- PMID: 31365590
- PMCID: PMC6668830
- DOI: 10.1371/journal.pone.0220671
High sporulation and overexpression of virulence factors in biofilms and reduced susceptibility to vancomycin and linezolid in recurrent Clostridium [Clostridioides] difficile infection isolates
Abstract
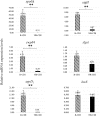
Clostridium [Clostridioides] difficile infection (CDI) is one of the leading causes of diarrhea associated with medical care worldwide, and up to 60% of patients with CDI can develop a recurrent infection (R-CDI). A multi-species microbiota biofilm model of C. difficile was designed to evaluate the differences in the production of biofilms, sporulation, susceptibility to drugs, expression of sporulating (sigH, spo0A), quorum sensing (agrD1, and luxS), and adhesion-associated (slpA and cwp84) pathway genes between selected C. difficile isolates from R-CDI and non-recurrent patients (NR-CDI). We obtained 102 C. difficile isolates from 254 patients with confirmed CDI (66 from NR-CDI and 36 from R-CDI). Most of the isolates were biofilm producers, and most of the strains were ribotype 027 (81.374%, 83/102). Most C. difficile isolates were producers of biofilm (100/102), and most were strongly adherent. Sporulation was higher in the R-CDI than in the NR-CDI isolates (p = 0.015). The isolates from R-CDI patients more frequently demonstrated reduced susceptibility to vancomycin than isolates of NR-CDI patients (27.78% [10/36] and 9.09% [6/66], respectively, p = 0.013). The minimum inhibitory concentrations for vancomycin and linezolid against biofilms (BMIC) were up to 100 times and 20 times higher, respectively, than the corresponding planktonic MICs. Expression of sigH, spo0A, cwp84, and agrD1 was higher in R-CDI than in NR-CDI isolates. Most of the C. difficile isolates were producers of biofilms with no correlation with the ribotype. Sporulation was greater in R-CDI than in NR-CDI isolates in the biofilm model of C. difficile. The R-CDI isolates more frequently demonstrated reduced susceptibility to vancomycin and linezolid than the NR-CDI isolates in both planktonic cells and biofilm isolates. A higher expression of sporulating pathway (sigH, spo0A), quorum sensing (agrD1), and adhesion-associated (cwp84) genes was found in R-CDI than in NR-CDI isolates. All of these factors can have effect on the recurrence of the infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Outcome of relapsing Clostridium difficile infections do not correlate with virulence-, spore- and vegetative cell-associated phenotypes.Anaerobe. 2015 Dec;36:30-8. doi: 10.1016/j.anaerobe.2015.09.005. Epub 2015 Sep 25. Anaerobe. 2015. PMID: 26403333
-
Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile.J Bacteriol. 2013 Feb;195(3):545-55. doi: 10.1128/JB.01980-12. Epub 2012 Nov 21. J Bacteriol. 2013. PMID: 23175653 Free PMC article.
-
An in silico evaluation of treatment regimens for recurrent Clostridium difficile infection.PLoS One. 2017 Aug 11;12(8):e0182815. doi: 10.1371/journal.pone.0182815. eCollection 2017. PLoS One. 2017. PMID: 28800598 Free PMC article.
-
Clostridioides difficile Biology: Sporulation, Germination, and Corresponding Therapies for C. difficile Infection.Front Cell Infect Microbiol. 2018 Feb 8;8:29. doi: 10.3389/fcimb.2018.00029. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29473021 Free PMC article. Review.
-
Clostridium difficile Biofilm.Adv Exp Med Biol. 2018;1050:97-115. doi: 10.1007/978-3-319-72799-8_7. Adv Exp Med Biol. 2018. PMID: 29383666 Review.
Cited by
-
Genetic Mechanisms of Vancomycin Resistance in Clostridioides difficile: A Systematic Review.Antibiotics (Basel). 2022 Feb 16;11(2):258. doi: 10.3390/antibiotics11020258. Antibiotics (Basel). 2022. PMID: 35203860 Free PMC article. Review.
-
Quorum sensing in human gut and food microbiomes: Significance and potential for therapeutic targeting.Front Microbiol. 2022 Nov 25;13:1002185. doi: 10.3389/fmicb.2022.1002185. eCollection 2022. Front Microbiol. 2022. PMID: 36504831 Free PMC article. Review.
-
Biofilm Formation of Clostridioides difficile, Toxin Production and Alternatives to Conventional Antibiotics in the Treatment of CDI.Microorganisms. 2023 Aug 26;11(9):2161. doi: 10.3390/microorganisms11092161. Microorganisms. 2023. PMID: 37764005 Free PMC article. Review.
-
Antibiotic Resistance and Biofilm Production Capacity in Clostridioides difficile.Front Cell Infect Microbiol. 2021 Aug 4;11:683464. doi: 10.3389/fcimb.2021.683464. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34422678 Free PMC article.
-
Treatment issues in recurrent Clostridioides difficile infections and the possible role of germinants.FEMS Microbes. 2020 Sep 23;1(1):xtaa001. doi: 10.1093/femsmc/xtaa001. eCollection 2020 Sep. FEMS Microbes. 2020. PMID: 37333958 Free PMC article. Review.
References
-
- McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–75. - PubMed
-
- Mac Aogain M, Moloney G, Kilkenny S, Kelleher M, Kelleghan M, Boyle B, et al. Whole-genome sequencing improves discrimination of relapse from reinfection and identifies transmission events among patients with recurrent Clostridium difficile infections. J Hosp Infect. 2015;90(2):108–16. 10.1016/j.jhin.2015.01.021 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

