Differential gene content and gene expression for bacterial evolution and speciation of Shewanella in terms of biosynthesis of heme and heme-requiring proteins
- PMID: 31362704
- PMCID: PMC6664582
- DOI: 10.1186/s12866-019-1549-9
Differential gene content and gene expression for bacterial evolution and speciation of Shewanella in terms of biosynthesis of heme and heme-requiring proteins
Abstract
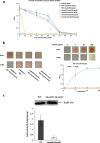
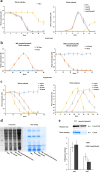
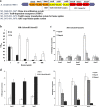
Background: Most species of Shewanella harbor two ferrochelatase paralogues for the biosynthesis of c-type cytochromes, which are crucial for their respiratory versatility. In our previous study of the Shewanella loihica PV-4 strain, we found that the disruption of hemH1 but not hemH2 resulted in a significant accumulation of extracellular protoporphyrin IX (PPIX), but it is different in Shewanella oneidensis MR-1. Hence, the function and transcriptional regulation of two ferrochelatase genes, hemH1 and hemH2, are investigated in S. oneidensis MR-1.
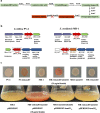
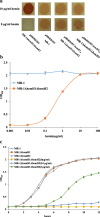
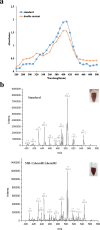
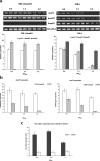
Result: In the present study, deletion of either hemH1 or hemH2 in S. oneidensis MR-1 did not lead to overproduction of extracellular protoporphyrin IX (PPIX) as previously described in the hemH1 mutants of S. loihica PV-4. Moreover, supplement of exogenous hemins made it possible to generate the hemH1 and hemH2 double mutant in MR-1, but not in PV-4. Under aerobic condition, exogenous hemins were required for the growth of MR-1ΔhemH1ΔhemH2, which also overproduced extracellular PPIX. These results suggest that heme is essential for aerobic growth of Shewanella species and MR-1 could also uptake hemin for biosynthesis of essential cytochrome(s) and respiration. Besides, the exogenous hemin mediated CymA cytochrome maturation and the cellular KatB catalase activity. Both hemH paralogues were transcribed in wild-type MR-1, and the hemH2 transcription was remarkably up-regulated in MR-1ΔhemH1 mutant to compensate for the loss of hemH1. The periplasmic glutathione peroxidase gene pgpD, located in the same operon with hemH2, and a large gene cluster coding for iron, heme (hemin) uptake systems are absent in the PV-4 genome.
Conclusion: Our results indicate that the genetic divergence in gene content and gene expression between these Shewanella species, accounting for the phenotypic difference described here, might be due to their speciation and adaptation to the specific habitats (iron-rich deep-sea vent versus iron-poor freshwater) in which they evolved and the generated mutants could potentially be utilized for commercial production of PPIX.
Keywords: Cytochrome; Ferrochelatase; Hemin; Protoporphyrin IX; Shewanella.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Differential Regulation of the Two Ferrochelatase Paralogues in Shewanella loihica PV-4 in Response to Environmental Stresses.Appl Environ Microbiol. 2016 Aug 15;82(17):5077-88. doi: 10.1128/AEM.00203-16. Print 2016 Sep 1. Appl Environ Microbiol. 2016. PMID: 27287322 Free PMC article.
-
An extracytoplasmic function sigma factor-dependent periplasmic glutathione peroxidase is involved in oxidative stress response of Shewanella oneidensis.BMC Microbiol. 2015 Feb 18;15:34. doi: 10.1186/s12866-015-0357-0. BMC Microbiol. 2015. PMID: 25887418 Free PMC article.
-
Co-ordination of iron acquisition, iron porphyrin chelation and iron-protoporphyrin export via the cytochrome c biogenesis protein CcmC in Pseudomonas fluorescens.Microbiology (Reading). 2003 Dec;149(Pt 12):3543-3552. doi: 10.1099/mic.0.26566-0. Microbiology (Reading). 2003. PMID: 14663086
-
N-Methyl Protoporphyrin IX: An Understudied Porphyrin.Chem Res Toxicol. 2022 Dec 19;35(12):2186-2193. doi: 10.1021/acs.chemrestox.2c00214. Epub 2022 Dec 2. Chem Res Toxicol. 2022. PMID: 36459538 Free PMC article. Review.
-
Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes.Mol Microbiol. 2007 Jul;65(1):12-20. doi: 10.1111/j.1365-2958.2007.05783.x. Mol Microbiol. 2007. PMID: 17581116 Free PMC article. Review.
Cited by
-
Production of Highly Active Extracellular Amylase and Cellulase From Bacillus subtilis ZIM3 and a Recombinant Strain With a Potential Application in Tobacco Fermentation.Front Microbiol. 2020 Jul 21;11:1539. doi: 10.3389/fmicb.2020.01539. eCollection 2020. Front Microbiol. 2020. PMID: 32793132 Free PMC article.
-
High-yield porphyrin production through metabolic engineering and biocatalysis.Nat Biotechnol. 2024 Jun 5. doi: 10.1038/s41587-024-02267-3. Online ahead of print. Nat Biotechnol. 2024. PMID: 38839873
References
-
- Qiu D, Xie M, Dai J, An W, Wei H, Tian C, Kempher ML, Zhou A, He Z, Gu B, et al. Differential regulation of the two Ferrochelatase paralogues in Shewanella loihica PV-4 in response to environmental stresses. Appl Environ Microbiol. 2016;82(17):5077–5088. doi: 10.1128/AEM.00203-16. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

