A Ciliary View of the Immunological Synapse
- PMID: 31362462
- PMCID: PMC6721628
- DOI: 10.3390/cells8080789
A Ciliary View of the Immunological Synapse
Abstract
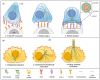
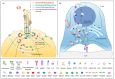
The primary cilium has gone from being a vestigial organelle to a crucial signaling hub of growing interest given the association between a group of human disorders, collectively known as ciliopathies, and defects in its structure or function. In recent years many ciliogenesis proteins have been observed at extraciliary sites in cells and likely perform cilium-independent functions ranging from regulation of the cytoskeleton to vesicular trafficking. Perhaps the most striking example is the non-ciliated T lymphocyte, in which components of the ciliary machinery are repurposed for the assembly and function of the immunological synapse even in the absence of a primary cilium. Furthermore, the specialization traits described at the immunological synapse are similar to those seen in the primary cilium. Here, we review common regulators and features shared by the immunological synapse and the primary cilium that document the remarkable homology between these structures.
Keywords: T lymphocytes; ciliary proteins; extraciliary functions; immunological synapse; primary cilium.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Primary Cilia Reconsidered in the Context of Ciliopathies: Extraciliary and Ciliary Functions of Cilia Proteins Converge on a Polarity theme?Bioessays. 2018 Aug;40(8):e1700132. doi: 10.1002/bies.201700132. Epub 2018 Jun 8. Bioessays. 2018. PMID: 29882973 Free PMC article. Review.
-
Primary cilia proteins: ciliary and extraciliary sites and functions.Cell Mol Life Sci. 2018 May;75(9):1521-1540. doi: 10.1007/s00018-017-2740-5. Epub 2018 Jan 5. Cell Mol Life Sci. 2018. PMID: 29305615 Free PMC article. Review.
-
The Rab GTPase Rab8 as a shared regulator of ciliogenesis and immune synapse assembly: From a conserved pathway to diverse cellular structures.Small GTPases. 2016;7(1):16-20. doi: 10.1080/21541248.2015.1111852. Epub 2015 Nov 20. Small GTPases. 2016. PMID: 26587735 Free PMC article.
-
The multifaceted roles of microtubule-associated proteins in the primary cilium and ciliopathies.J Cell Sci. 2023 Dec 1;136(23):jcs261148. doi: 10.1242/jcs.261148. Epub 2023 Dec 14. J Cell Sci. 2023. PMID: 38095645 Review.
-
Regulation of the Extracellular Matrix by Ciliary Machinery.Cells. 2020 Jan 23;9(2):278. doi: 10.3390/cells9020278. Cells. 2020. PMID: 31979260 Free PMC article. Review.
Cited by
-
ARL3, a small GTPase with a functionally conserved role in primary cilia and immune synapses.Small GTPases. 2021 May;12(3):167-176. doi: 10.1080/21541248.2019.1703466. Epub 2019 Dec 18. Small GTPases. 2021. PMID: 31826708 Free PMC article. Review.
-
The Immune Checkpoint Protein PD-L1 Regulates Ciliogenesis and Hedgehog Signaling.Cells. 2024 Jun 8;13(12):1003. doi: 10.3390/cells13121003. Cells. 2024. PMID: 38920633 Free PMC article.
-
The exocyst complex and intracellular vesicles mediate soluble protein trafficking to the primary cilium.Commun Biol. 2024 Feb 21;7(1):213. doi: 10.1038/s42003-024-05817-2. Commun Biol. 2024. PMID: 38378792 Free PMC article.
-
Primary Ciliary Signaling in the Skin-Contribution to Wound Healing and Scarring.Front Cell Dev Biol. 2020 Nov 13;8:578384. doi: 10.3389/fcell.2020.578384. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33282860 Free PMC article. Review.
-
Lymphocyte Polarization During Immune Synapse Assembly: Centrosomal Actin Joins the Game.Front Immunol. 2022 Feb 11;13:830835. doi: 10.3389/fimmu.2022.830835. eCollection 2022. Front Immunol. 2022. PMID: 35222415 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

